Ventilator Associated Pneumonia in an Infant Caused by Stenotrophomonas maltophila – A Case Report
Neelima Angaali1, Nina Dutta Roy2, Sudharshan Raj Chitgupikar3, Preeti Subramanian4, Jaya Lakshmi Pabbati5
1 Associate Professor, Department of Microbiology, MediCiti Institute of Medical Sciences (MIMS), Hyderabad, Telangana, India.
2 Head of Department, Department of Microbiology, Pathcare Labs, Hyderabad, Telangana, India.
3 Associate Professor, Department of Pediatrics, MIMS, Hyderabad, Telangana, India.
4 Assistant Professor, Department of Pediatrics, MIMS, Hyderabad, Telangana, India.
5 Assistant Professor, Department of Pediatrics, MIMS, Hyderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Neelima Angaali, H.No.-3-6-536, Raja Residency, Flat-201, Street-7, Himayat Nagar, Hyderabad-500029, India.
E-mail: neelimasudharshan@gmail.com
Stenotrophomonas maltophila (S.maltophila) is an aerobic Gram-negative bacillus that is a frequent colonizer of fluids used in the hospital setting. The organism is known to cause life threatening infections in immuno-compromised patients especially in those who are neutropenic, on chemotherapy or on broad spectrum antibiotics.
We report a case of ventilator associated pneumonia caused by Stenotrophomonas maltophila in a two-month old infant who later developed multi organ dysfunction syndrome.
In seriously ill paediatric patients, S.maltophila should also be considered as a possible pathogen for Ventilator-Associated Pneumonia (VAP), hence empiric antibiotic choice should include antimicrobials that are active against S. maltophila. An early identification and treatment of VAP with Multi-Drug Resistant (MDR) strains with appropriate antibiotics has a significant impact on morbidity and mortality.
Cefotaxime, Endo Tracheal Aspirate, Hypotension, Multi Drug Resistant Strains
Case Report
A two-month old female infant presented to the Emergency department of Mediciti Institute of Medical Sciences in an unresponsive state following excessive cry that lasted 1-2 hours. On clinical examination baby was gasping, with ongoing convulsions, cyanosed with compromised cardiovascular status. Child was stabilized, intubated and connected to ventilator. Blood culture was taken and she was started on intravenous cefotaxime at a dose of 200mg/kg/day in three divided doses, antiepileptics and inotropic support. There was no history of head trauma, fever, refusal of feeds, and vaccination in recent past. Blood samples were taken for Renal & Liver Function Tests (RFT & LFT), serum electrolytes, C-reactive protein (CRP), Complete blood counts and blood culture. Chest X-ray AP view was taken which revealed patchy opacities in upper and lower zones of right lung.
RFT & LFT were within normal limits, CRP was 3.7mg/L, Haemoglobin (Hb): 9.3gm%, and WBC: 30000/cu.mm.
Culture and antibiotic sensitivity was done by conventional and automated (BD PHOENIX) methods. Endotracheal aspirate was cultured aerobically on sheep blood agar, chocolate agar, Mac Conkey agar and nutrient agar media. Culture media were incubated at 37°C for 24-48 hours. After incubation, identification of bacteria from positive cultures was done with standard microbiological technique which includes Gram’s staining and biochemical reactions [1]. All the isolates were subjected to antibiotic sensitivity by Kirby Bauer disc diffusion method. The antibiotic sensitivity was done according to the Clinical Laboratory Standards Institute (CLSI) guidelines [2].
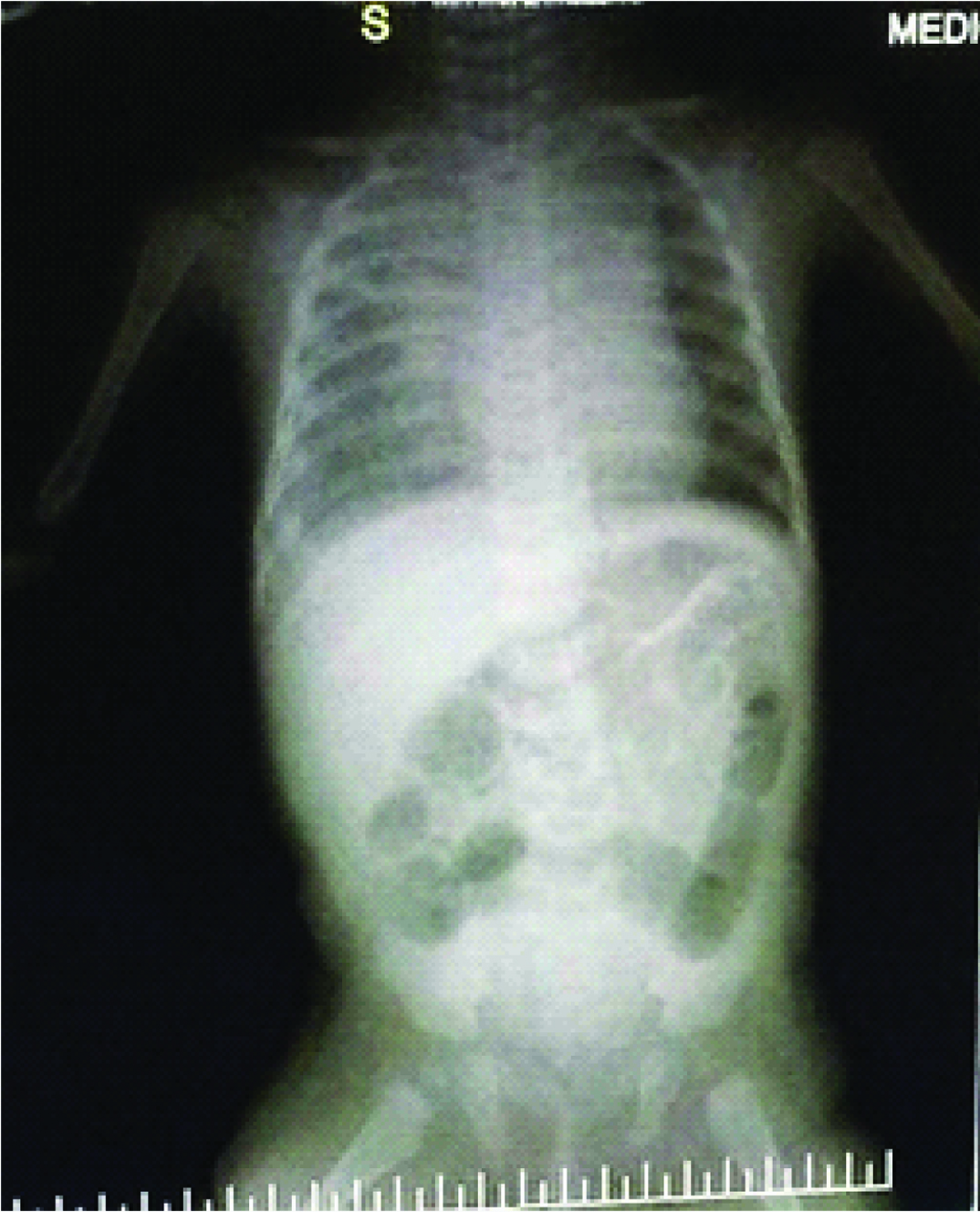
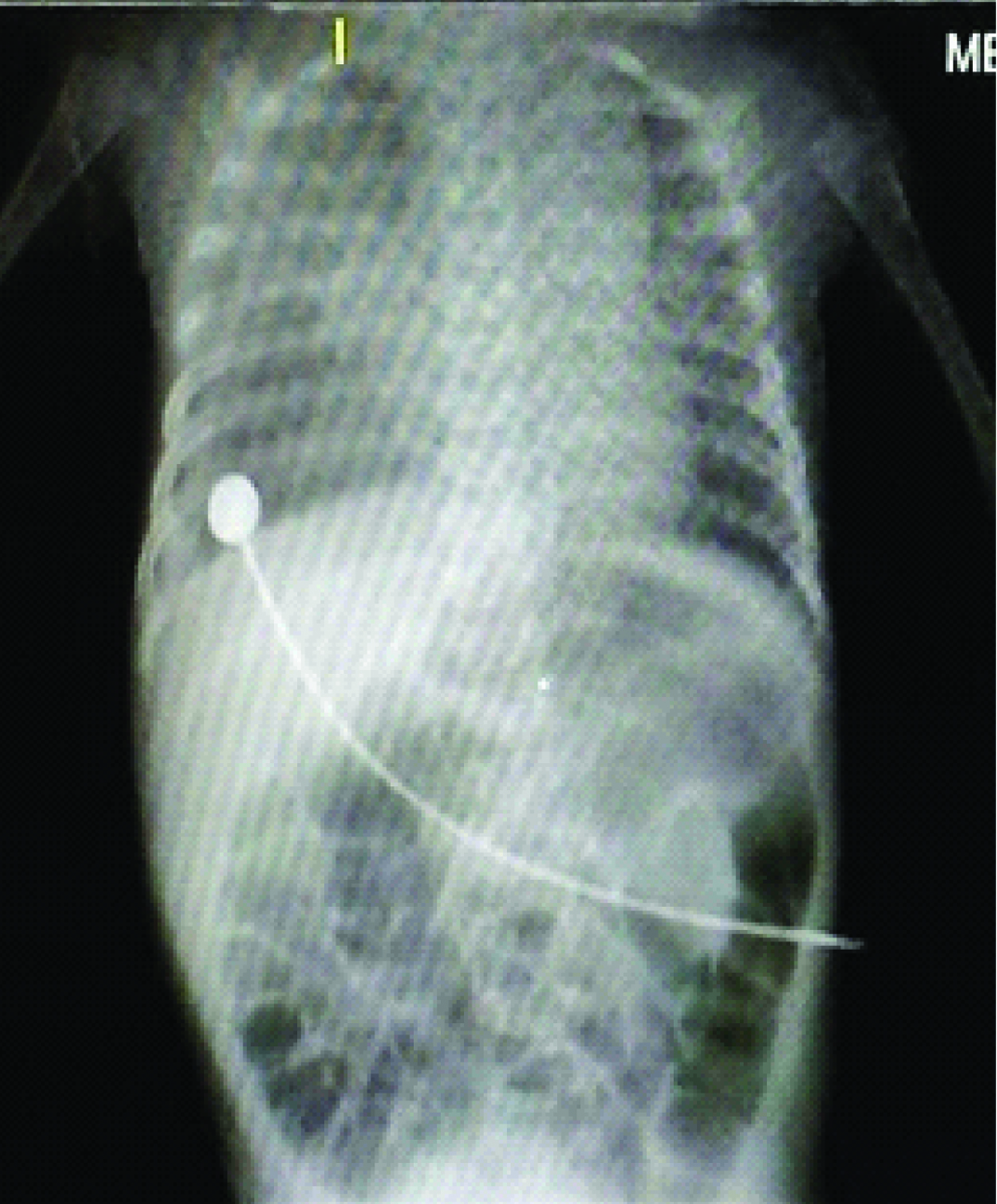
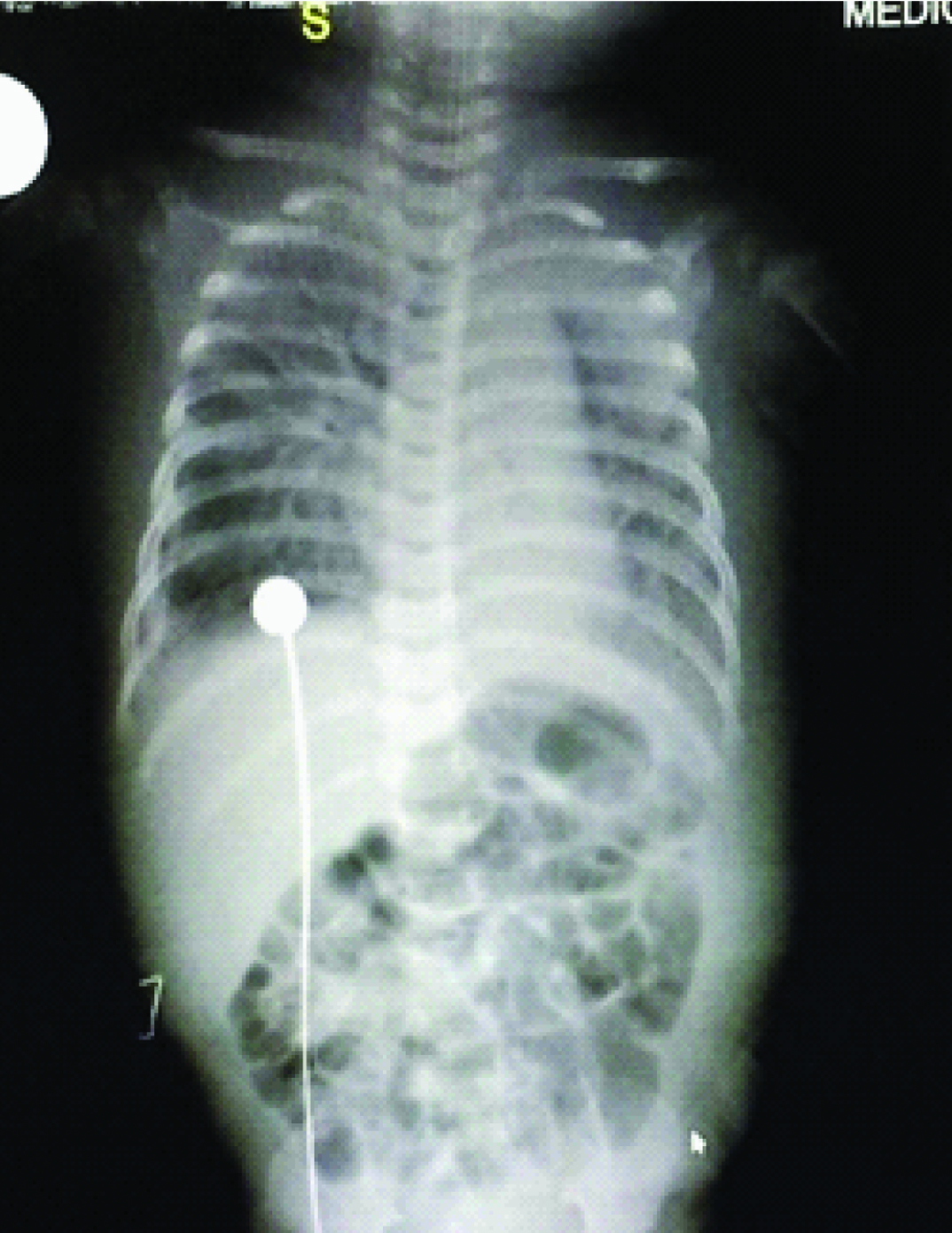
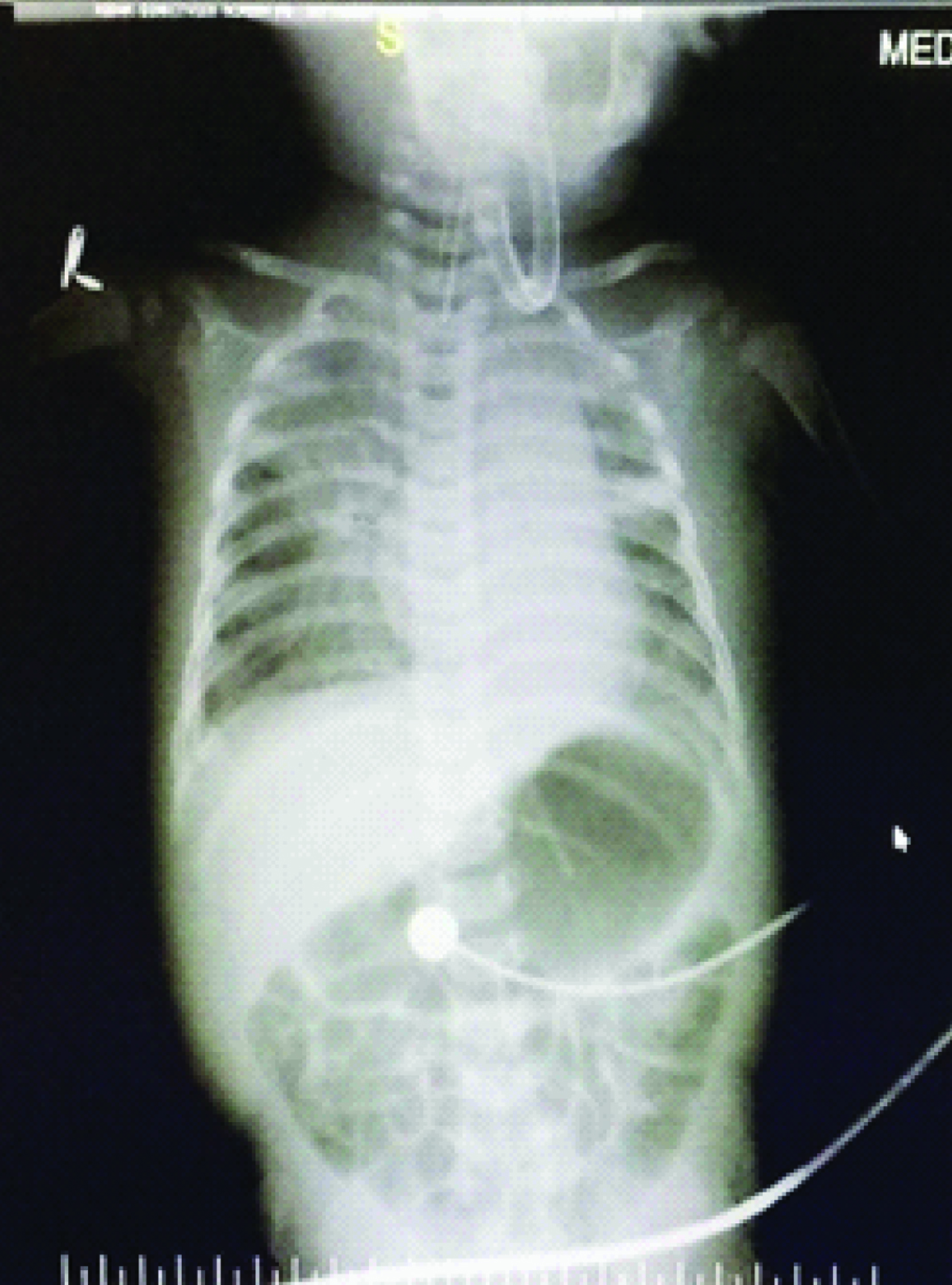
Day 2 and day 3 [Table/Fig-1,2] chest X-Ray’s showed persistence of previous findings and provisional report of blood culture was negative. Repeat CRP on day 4 was elevated (45.2mg/L). Chest X-Ray on day 4 [Table/Fig-3] revealed diffuse infiltrates in right upper and middle lobes. On day 5 Endotracheal Tube (ET) was changed in view of increasing secretions and ET aspirates were sent for culture and sensitivity. A provisional diagnosis of VAP was arrived at by above clinical and radiological appearance. Hence antibiotics were changed to Inj. Meropenem at 20mg/kg/dose intravenous (IV) TID and Inj. Linezolid 10 mg/kg/dose IV TID. Confirmed blood culture report was available on day 6 which revealed no growth. Child continued to have increased ET secretions with requirement of augmented ventilator and inotropic support. Chest X-Ray on day 7 [Table/Fig-4] revealed homogenous opacity forming in the lower lobe of the right lung. But she stayed clinically seizure free since the initial presentation till day 8. By day 9 ET aspirate culture and sensitivity showed growth of Stenotrophomonas maltophila sensitive to cotrimoxazole and ciprofloxacin.
Gram stain showed few pus cells >10/High-Power Field (HPF)and Gram-negative Bacilli (GNB) which were straight measuring 0.5-1.5μm arranged in singles were seen. Culture showed >105 colony-forming unit (CFU)/ml on blood agar, and non-lactose fermenting colonies on Mac Conkey agar. The colonies were smooth, glistening with entire margins. It was catalase positive, oxidase negative, motile, citrate utilized, nitrate reduction positive, DNAse positive, Oxidative Fermentative (OF) maltose positive, sensitive to colistin. Later it was confirmed with BD Phoenix automated ID susceptibility system.
Antibiotics were changed to IV ciprofloxacin 10mg/kg/dose TID and IV flucanazole 3mg/kg/day was also added. IV fluconazole was added as fungal infections are common in children treated with long term broad spectrum antibiotics. Child started to have recurrence of seizures. On day 10 she developed hyponatremia and anasarca. She progressively deteriorated and developed petechiae and bleeding from puncture sites with refractory hypotension on day 11. IV noradrenaline infusion was added. Despite the above efforts child succumbed to the illness on day 12 of admission.
Discussion
Stenotrophomonas maltophila is a non-fermentative Gram-negative bacillus, previously known as Pseudomonas maltophila and later Xanthomonas maltophila usually found in water, soil, plants, food and fluids in hospital settings [3–5]. It is an emerging pathogen as a significant cause of nosocomial infections particularly among severely debilitated and immuno-compromised patients and more in those who are neutropenic, on chemotherapy, those receiving long-term antimicrobial therapy and in individuals with indwelling central venous catheters etc [3–7]. This organism has gained a lot of interest in the recent times due to increased recognition of its pathogenic potential and marked antibiotic resistance [3]. Ventilator-Associated Pneumonia (VAP) is defined as pneumonia acquired in ventilated patients who are intubated for more than 48 hours [8]. Other qualifying signs and symptoms are presented in [Table/Fig-5] [9].
Diagnostic criteria of VAP [9].
| 1. | Presence of new, persistent pulmonary infiltrates, not otherwise explained, appearing on chest radiographs |
| 2. | Temperature above 380C or lower than 360C |
| 3. | Leucocytosis or leucopenia (more than 10,000 or less than 4,000 cells/mm3), |
| 4. | Purulent respiratory secretions |
| 5. | Endotracheal aspirate (ETA) culture - > 105 CFU/ml |
Clinical and microbiological diagnosis remains challenging due to its varied clinical presentation and expectation from microbiology for its accurate, relevant and timely bacteriological evidence [10].
Although Bronchoalveolar Lavage (BAL), lung biopsy, Protected Specimen Brush (PSB) and mini-BAL are regarded as the best methods for the diagnosis of VAP, with high specificity and sensitivity, various studies have also demonstrated the efficacy of quantitative culture of Endotracheal Aspirate (ETA) as a diagnostic method in intubated patients [11].
Duration of mechanical ventilation influences the type of organism that causes VAP [12]. In general, late onset VAP is caused by multi-drug resistant bacteria and the ones which are more difficult to treat whereas early VAP is caused by pathogens that are sensitive to antibiotics [13,14].
This was a typical case of VAP on day 7. Culprits of organisms causing late VAP are typically Multi Drug Resistant (MDR) bacteria, such as Acinetobacter, Pseudomonas aeruginosa, Methicillin-Resistant Staphylococcus aureus (MRSA), and Extended-Spectrum Beta-Lactamase Producing Bacteria (ESBL) [13]. MDR organisms have varied prevalence between institutions and also within institutions [14]. The incidence of VAP due to Stenotrophomonas maltophila is 1.7% [14].
Stenotrophomonas is inherently resistant to β lactams such as penicillins, cephalosporins, carbapenems and aminoglycosides and is typically sensitive to co-trimoxazole and quinolones [15].
In this case too it was sensitive to co-trimoxazole and ciprofloxacin. Prior antibiotic therapy, >7days duration of mechanical ventilation and use of broad spectrum antibiotics are some of the risk factors that can cause VAP due to MDR organisms [16]. Delay in the initiation of relevant antimicrobial therapy increases the VAP mortality rate as was also seen in our case [11,13–15], a higher mortality rate has been observed in patients who did not receive appropriate therapy or received it after a delay, in comparison to those who received appropriate therapy [17].
Conclusion
S.maltophila should also be considered as a likely pathogen in clinical setting of VAP especially in critically ill paediatric patients. Empiric antibiotic therapy choice should include agents that are active against S. maltophila in patients who are deteriorating on broad spectrum β-lactam antimicrobial agent. Correct antibiotic therapy instituted with proper monitoring at the right time, can be lifesaving so for that diagnosis of VAP with multi-drug resistant strains as early as possible is of prime importance.
[1]. John Gerald collee, Tests for identification of bacteria. Mackie and Mc Cartney 1996 14th editionElsevier Health Sciences:131-149. [Google Scholar]
[2]. Clinical and laboratory standards institute. Methods for disk susceptibility tests for bacteria that grow aerobically, 7th edition, document m2–a8. Wayne, pa: clinical and laboratory standards institute 2005 [Google Scholar]
[3]. Nicodemo AC, GarcíaPaez JI, Antimicrobial therapy for Stenotrophomonas maltophila infectionsEur J Clin Microbiol Infect Dis 2007 26:229-37. [Google Scholar]
[4]. Yemisen M, Mete B, Tunali Y, Yentur E, Ozturk R, A meningitis case due to Stenotrophomonas maltophila and review of the literatureInt J Infect Dis 2008 23:1-3. [Google Scholar]
[5]. Teo WY, Chan MY, Lam CM, Chong CY, Skin manifestation of Stenotrophomonas maltophila infectionAnn Acad Med Singapore 2006 35(12):897-900. [Google Scholar]
[6]. Lo WT, Wang CC, Lee CM, Chu ML, Successful treatment of multi-resistant Stenotrophomonas maltophila meningitis with ciprofloxacin in a pre-term infantEur J Paediatr 2002 161:680-82. [Google Scholar]
[7]. Denis F, Sow A, David M, Etude de deuxcas de meningitis a Pseudomonas maltophilia observes au SenegalBull Soc Med Afr Noire Lang Fr 1977 22:135-39. [Google Scholar]
[8]. Marc J, Bonten M, Kollef MH, Hall JB, Risk factors for ventilator-associated pneumonia: from epidemiology to patient managementCID 2004 38:1141-49. [Google Scholar]
[9]. Rello J, Artur J, Baraibar J, International conference for the development of consensus on the diagnosis and treatment of ventilator associated pneumoniaChest 2001 120:955-70. [Google Scholar]
[10]. Cook D, Mandel L, Endotracheal Aspiration in diagnosis of VAPChest 2000 117:195-97. [Google Scholar]
[11]. Bergmans DC, Bonten MJ, De Leeuw PW, Stobberingh EE, Reproducibility of quantitative cultures of endotracheal aspirates from mechanically ventilated patientsJ Clin Microbiol 1997 35:796-98. [Google Scholar]
[12]. Kalanuria AA, Zai W, Mirski M, Ventilator associated pneumonia in the ICUCrit Care 2014 18(2):208-15. [Google Scholar]
[13]. Hunter JD, Ventilator associated pneumoniaBMJ 2012 344:e3325 [Google Scholar]
[14]. American Thoracic Society, Infectious Diseases Society of AmericaGuidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumoniaAm J Respir Crit Care Med 2005 171(4):388-416. [Google Scholar]
[15]. Winn W, Koneman E, Non fermentative gram negative bacilliKoneman’s color atlas and text book of diagnostic Microbiology 2005 6th editionLippincott Williams and Wilkins:300-391. [Google Scholar]
[16]. Trouillet JL, Chastre J, Vuagnat A, Ventilator associated pneumonia caused by potentially drug-resistant bacteriaAm J Respir Crit Care Med 1998 157:531-39. [Google Scholar]
[17]. Mukhopadhyay C, Krishna S, Vandana KE, Shenoy A, Bairy Ventilator-Associated Pneumonia with Col-S Strains: A successful comeback of colistin!The Brazilian Journal of Infectious Diseases 2008 12(5):444-46. [Google Scholar]