Introduction
Acute myocardial infarction and sudden cardiac death are the leading cause of mortality in adults worldwide. In most cases, the underlying pathology for these syndromes begins with the process of atherosclerosis. Here, the atherosclerosis can be developed as a state of low-grade inflammation of the intima (inner lining) of the coronary arteries. Gradually, the process is accelerated by certain well-established risk factors such as hypertension, dyslipidemia, diabetes, smoking, and genetics. In the case of coronary atherosclerosis, this slow progression leads to the gradual thickening of the inner layer of the coronary arteries, which may, over time narrow the lumen of the artery to various degrees and may lead to total or near total coronary occlusion [1]. In this regard, a CTO in coronary artery is recognized by a significant atherosclerotic plaque burden within the artery, resulting in complete (or nearly complete) occlusion of the vessel and absence of any antegrade flow beyond the coronary occlusion (TIMI-0 flow). Although the duration of the occlusion is difficult to determine on clinical grounds, a total occlusion must be present for at least three months to be considered a true CTO [2]. Thus, CTO lesions are defined as the coronary lesions with TIMI-0 flow, within the occluded segment along with angiographic or clinical evidence of occlusion duration >3months. They account for about one-third of the coronary lesions [3,4]. Despite of high prevalence, only 8%–15% of the patients undergo (PCI) [5–7]. This disparity between the frequency of the CTO and percutaneous treatment truly represents CTO as one of the “final frontier” in interventional cardiology.
CTO poses a management dilemma for the interventional cardiologists due to the technical/procedural complexities (i.e., perforations, renal failure, bleeding, or peripheral vascular injury) and clinical uncertainties (i.e., urgent coronary artery bypass grafting, myocardial infarction, or death). Since clear evidence demonstrating clinical benefits by percutaneous recanalization of CTO were lacking, majority of such patients were treated medically or surgically until recently. Lately, the body of published evidences have clearly demonstrated the clinical benefits with successful recanalization of CTO lesions. Studies have also demonstrated that recanalization of CTO lesions offers the benefits of improved anginal status, improved left ventricular ejection fraction, reduced the need for surgical revascularization, long-term patency, improved quality of life, and enhanced survival [8–12]. Apart from remarkable clinical benefits to the patients, improved procedural success rate has also raised an interest into this field. Earlier, the technical success for PCI of CTO was limited in 40%–57% [13–16]. However, due to advancement of guidewires, devices, and techniques in recent years, successful recanalization may now be achieved in as many as 80% of the CTO lesions [17–19]. Hence, it becomes important for interventional cardiologists to be acquitted and updated with contemporary management techniques for CTO lesions. With this background, the details of current devices and techniques available for recanalization of CTO are summarized in the present review, which will provide valuable information for fellow interventional cardiologists.
PERCUTANEOUS RECANALIZATION OF CHRONIC TOTAL OCCLUSION: TECHNICAL CONSIDERATION
Guiding catheter: The selection of appropriate guiding catheter that provides maximal backup and support is crucial [20]. Most operators generally prefer femoral artery access and utilization of 7–8 Fr guiding catheter. Here, the 7–8 Fr guiding catheter provides greater support, allows insertion of covered stent grafts (in case of perforation which is commonly encountered complication in CTO recanalization), and allows the use of simultaneous dual Over the Wire (OTW) catheters or the use of Intravascular Ultrasound (IVUS) beside an OTW balloon. The 6 Fr guiding catheters are also used especially in cases of short occlusions; lesions at the proximal segment of the target vessel, or by operators skilled with active guide manipulation. EBU catheters (i.e., Voda Left, extra back-up, Amplatz Left catheters) are preferable for lesions in the left coronary system. Judkins-type guide catheters provide relatively inferior support and are associated with reduced success with hard fibro-calcific occlusions. For lesions in RCA, the Judkins right catheter is frequently used as it provides deep intubation and minimal risk of ostial dissection. The back-up support for catheter can be improved with the use of an anchoring balloon (1.5mm–2.0mm balloon inflated at low pressure in a wired side branch proximal to the CTO lesion). In addition, the use of Left Amplatz 0.75–2 shapes or hockey stick shaped catheter can offer improved back-up support for RCA lesions, but are associated with risk of dissection [3].
Microcatheters and OTW balloons: OTW balloon or microcatheter is used in PCI of CTO in order to ease torque in the tip response, preventing flexion, kinking or prolapse of guidewire as well as improving penetration ability by providing additional support to the shaft of the wire. They also allow the exchange of one guidewire for another without sacrificing the progress made through the lesion. Since microcatheters improve wire manipulation due to their larger inner lumen and hydrophilic coating, they are preferred over OTW balloons in certain instances. More flexible tip and radiopaque marker at very distal end of the catheter avoid too far advancement into lesion. Braided microcatheters prevent shaft kinking especially while crossing tortuous vessels. Despite numerous advantages, the expense involved limits its use. Finally, the choice between OTW balloon and microcatheter depends upon complexity of the lesion and operator’s experience. Here, the most widely used microatheters in routine PCI of CTO are described with a focus on extensively used microcatheters rather than on recently developed microcatheters available [21].
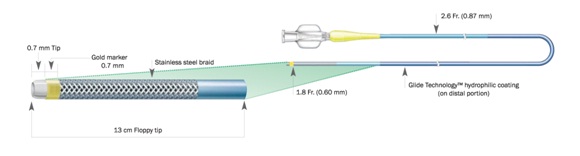
1. Finecross: Finecross, a unique and innovative lower profile microcatheter, is designed for highly stenosed lesions of distal tortuous vessels [Table/Fig-1]. Currently, it is available in two lengths (130cm and 150cm) which enable the operator to treat proximal CTOs via a retrograde approach [21]. The catheter tapers from 2.6 Fr to 1.8 Fr which allows better lesion crossability. The unique features of the catheter are hydrophilicity, flexibility, and a very distal tip marker. The hydrophilic coating of Polytetrafluoroethylene (PTFE) allows passage through small vessels as well as distal segment of tortuous vessels with less resistance. As the radiopaque marker located at very distal tip (0.7mm from the tip), the exact progress through the lesions can be identified [22].
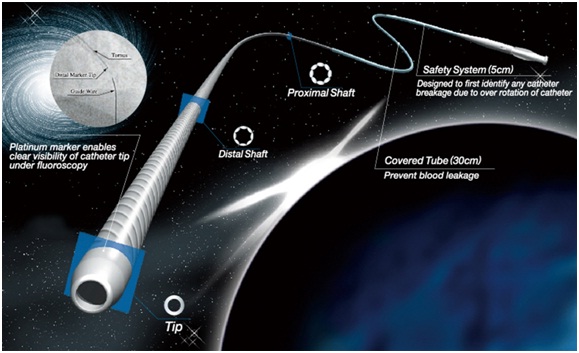
2. Tornus: Tornus (Asahi Intecc, Japan) microcatheter is a braided-wire mesh OTW microcatheter with left-handed thread [Table/Fig-2]. The catheter consists of eight individual stainless steel wires (0.007" diameter) spirally bound together to form a tapered microcatheter. The catheter is advanced by screwing technique. It is recommended to release the torque energy after 20 rotations to avoid wire fractures. The screwing of the catheter transmits rotational energy to the tip and thereby create pathway. Its length is enough for antegrade procedures but not for retrograde procedure. It is useful for crossing resistant occlusion. The disadvantage of this catheter is the cost which is near that of a drug-eluting stent [21–23].
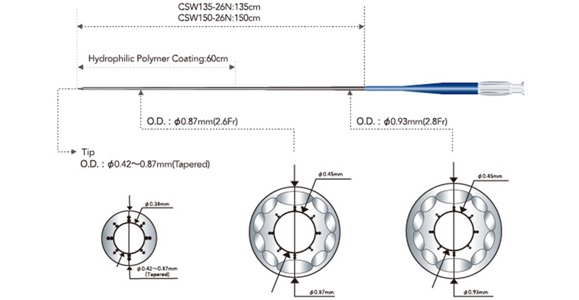
3. Corsair: Corsair (Asahi Intecc, Japan) is a recently developed microcatheter to facilitate crossing in tortuous vessels and highly stenosed lesions. It consists of a 2.7 Fr catheter with a braiding of 10 wires interwoven to form a metallic tube with a length of 135cm and 150cm [Table/Fig-3]. Two strips of thick stainless steel and eight strips of thin stainless steel provide screw-head support to the distal portion of the catheter. The characteristic features are hydrophilic polymer (which covers distal 60 cm) and tapered, tungsten powered soft tip. Hydrophilic polymer provides enhanced manoeuvrability while tapered and tungsten powered soft tip provides good tip flexibility. The device employs similar screwing action to the Ashahi Tornus catheter and its sturdy nature straightens the vessels. This device is specially designed to cross collateral channels. It can also be used in the antegrade direction for wire support and exchange as well as lesion crossing [21–23].
4. Venture: The Venture Catheter (St Jude Medical, USA) is a low-profile OTW support catheter. It consists of 6 Fr compatible and torqueable catheters with radiopaque atraumatic tip. It is specially designed to cross lesions with difficult angles. The unique deflectable tip catheter provides directional steering control and focused push ability for efficient wire placement [24].
Guidewires: Guidewire selection is the most critical phase of CTO recanalization as it determines the technical success or failure. The important components of CTO guidewire are tip load, tip stiffness, guidewire flexibility, ability to shape, shaping memory, shaft support, torque transmission, track ability and resistance to trapping of the wire within the occlusion [22]. Currently available guidewires are broadly divided into two groups: Hydrophilic (Polymer-coated or lubricious guidewires) and Non-hydrophilic (Conventional or non-coated guidewires). Both groups can be further sub-divided into those with non-tapered tips (0.014", standard gauge) and those with tapered tips (0.008–0.010") [25].
1. Hydrophilic guidewires (Polymer-coated or lubricious guidewires): They provide good manoeuvrability in tortuous and long vessels. Being lubricant, they can be advanced with minimal resistance and may be steered more easily in true lumen immediately after sharp bend. They are more likely to penetrate beneath plaque and dissect the vessel. Apart from limited tactile feel, lack of optimal tip control is another limitation of the hydrophilic guidewires. They have tendency to create large false lumen. Hence, hydrophilic wires are considered as a last resort for total occlusions. However, operators select hydrophilic-coated wires for CTOs when there is tortuous or fibrocalcific anatomy proximal to occlusion. There are two different groups of hydrophilic wire. Whisper MS and LS, Pilot 50, Fielder/Fielder FC, Fielder X-Treme, Shinobi and Shinobi Plus are the hydrophilic guidewires which have hydrophilic coating only on the tip, while the Pilot 150/200, Choice PT/PT2, PT Graphix/Graphix P2, Runthrough Hypercoat and Hydr’8 have hydrophilic coating on the shaft as well as the tip [25,26].
2. Non-hydrophilic guidewires (Conventional, non-coated guidewires): They are characterized by better tactile feel and a more controlled torque response. Hence, they are less likely to dissect the vessel. These wires tend to encounter more resistance inside the lumen. [25]. Hence, some uncoated, spring-coil wires are designed with a tapered-tip design (i.e., Cross-It, Persuader 9 g, Confianza, and Confianza Pro). Certain guidewires have greater tip stiffness, rather than sharpness, to increase their penetration capabilities (i.e., Magic S 9 g and Magic Ex 18 g [27]. The most widely used CTO guidewires are described below.
a. Fielder: The guidewires of Fielder family are designed with excellent balance of lubricity, support, and torque response. The hydrophilic coating (SLIP COAT® over tip the spring coil and PTFE coating over shaft) provides lubrication, track-ability and torque transmission. The Fielder-FC is developed to facilitate device delivery through tortuous anatomy with enhanced intermediate support. The gentle tip allows easy navigation through tortuous anatomy and at the same time potentially less traumatic to the vessel wall. The Fielder XT, a soft and tapered tip (0.009") guidewire, is useful for the treatment of complex lesions. Recently, Fielder XT-A and Fielder XT-R have been developed, which provide potential for the loose tissue and collateral channel tracking by a small curve and tapered tip [25,28].
b. Gaia: The guidewires of Gaia family are gaining acceptance for treating CTO lesions in recent years. Due to long hydrophilic coating, they can be manipulated smoothly when used in conjunction with a support catheter. Double coil structure of Gaia guidewires transfer torque force to the distal and round core design to the distal end eliminates the “Whip motion” phenomenon. Because of excellent torque transmission, they are very effective for retrograde wire crossing and reverse CART. The micro-cone tip of Gaia wires provide better penetration and allow the wire to penetrate more easily in hard lesions while keeping a flexible tip. Gaia First (0.10") is a new tapered guidewire and is gaining acceptance. The Gaia second guidewire (0.011") is designed with excellent torque transmission and is particularly efficient at passing tortuous arteries and entering fine channels [25,28].
Different Guidewire Strategies for Refractory CTO Recanalization
Antegrade approach: Most operators first approach CTO with the same floppy guidewires used for PCI in non-occluded lesions. CTOs which cannot be crossed with floppy wires can be encountered with progressively stiffer wires. CTOs are usually approached in combination with OTW balloons or microcatheter for the exchange of the floppy wires with stiffer guidewires, transmission of the torque to the tip and improved feedback. By gradually choosing stiffer guidewires with higher tip loads, vessel perforation can be minimized. However, tactile feel of the guidewire decreases as stiffness of the tip increases [21,27,29]. The shaping of guidewire tip is kept as short as possible in length (1.0mm-2.0mm from the tip of guidewire) with angle 40–50 curve initially. This manipulation of guidewire tip is important to penetrate proximal fibrous cap. A gentle secondary curve (i.e., 15–20 curve at 3mm–4mm proximal to distal tip) is necessary to navigate into CTO body to orient tip and to cross long distal fibrous cap in hard spring coil wires [3,21].
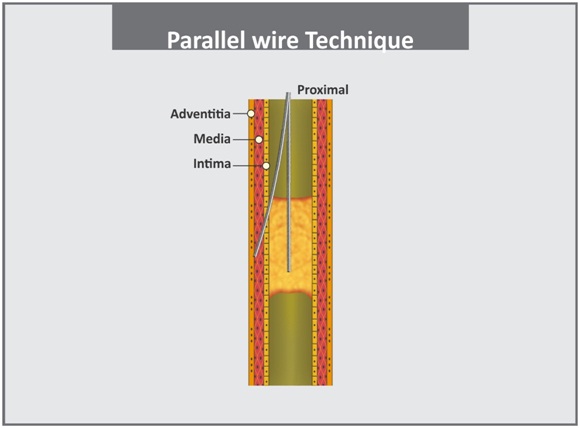
a. Parallel wire technique: Parallel-wire technique was first described by Reifart et al., [3]. In the parallel-wire technique, when a wire enters a false lumen, it is left in place, and a second wire (typically stiffer and tapered with different tip bend) supported by an OTW balloon catheter is passed along the same path parallel to the first wire, with care taken to avoid wire twisting. If guidewire enters false lumen while encountering CTO, vigorous attempts to redirect it into true lumen often creates extramural hematoma which may lead to collapse of the proximal cap and thereby make lumen re-entry impossible. Leaving the wire in place marks the dissection channel, guide the second wire by occluding wrong pathway as well as straighten the bend in the vessel [Table/Fig-4] [20,27].
b. See-saw wiring technique: This technique is a variation of the parallel wire technique. In the parallel-wire technique, if the second guidewire also fails to enter the distal true lumen, it is left in place and the first wire is withdrawn, steered in the direction of true-lumen. Here, both wires are supported by OTW angioplasty catheters and are alternatively used to probe the occlusion to find the true lumen. Sometimes, three or more wires are used to identify true lumen [21,27].
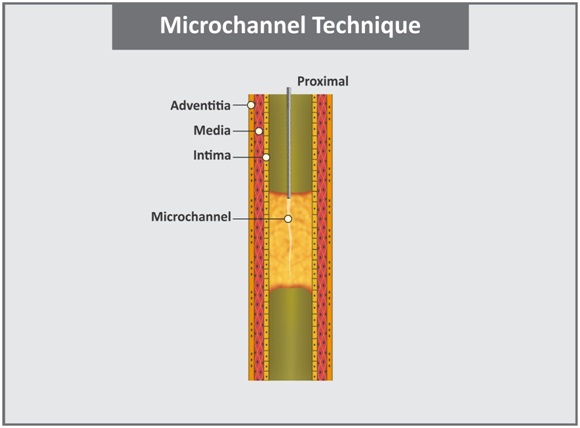
c. Microchannel technique: This technique was proposed by Carlino for straight CTO lesion with concave proximal cap, based upon histological CTO data [30]. Histological examination of CTOs have identified that majority of CTOs have intraluminal microchannels, varying in size from 100μm –500μm which run within and parallel to the thrombosed parent vessel [31,32]. They are distinct from the vasa vasora, which run principally in a radial direction. Strauss et al., have proposed that these intraocclusion microvessels may provide a route for the guidewire to cross the CTO [33]. In this technique [Table/Fig-5], proximal fibrous cap is first centrally penetrated to 1mm–2mm with very stiff guidewire with advancement of an OTW balloon or microcatheter with subsequent careful injection of nitrates (50μg–100μg) and undiluted contrast (1mL). It is critical that the puncture of the proximal fibrous cap is performed with the guidewire tip in perpendicular fashion to avoid injecting into the subadventitial space. Contrast injection immediately distal to proximal cap of CTO identifies and enlarges microchannels creating a pathway between proximal and distal true lumens [30].
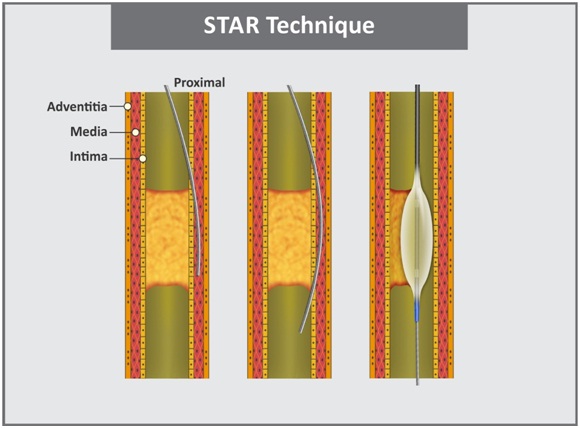
d. Star technique: The STAR technique was initially described by Colombo et al., [34], it involves use of knuckle wire, usually formed by pushing hydrophilic guidewire until it forms a tight loop at its tip once the wire is within dissection flap [Table/Fig-6]. The knuckle wire is advanced continually until it spontaneously re-enters true lumen. Here, the applied force is evenly distributed over a large surface area, along the length of the loop which facilitates communication with true lumen. Subsequently, Carlino introduced modified STAR technique which is known as “contrast-guided STAR” [35]. In this modified technique, injection of pure contrast is given into the subintimal space via an OTW balloon or a microcatheter. Contrast injection in the dissection plane occasionally extends the dissection plane back into the true lumen, allowing the successful completion of the procedure. However, it may prove hazardous and hence, this technique should be performed by experienced operators in patients with refractory symptoms and no alternative low-risk options for revascularization. Galassi further refined STAR technique by proposing “mini-STAR” using very soft Fielder polymeric guidewire [36]. In contrast to the STAR and guided-STAR technique, in which an “umbrella handle” wire configuration is made prior to insertion though the microcatheter, in the “mini-STAR” technique two curves are placed on the wire, a small first curve (40°–50°) at the distal end (1mm–2mm proximal to the tip) and second curve (15°–20°) 3mm–5mm proximal to the tip. The wire is advanced towards the CTO resulting in distal true lumen crossing or (in case of channel interruption or due to the presence of harder tissue) the wire automatically forms a J-loop that is advanced for subintimal penetration of the CTO, followed by efforts to re-enter the true lumen as proximally as possible. This J-tip is smaller compared to the one that is created in STAR technique and thereby creation of minimal subintimal spaces. The authors reported success rate of 97.6%. The procedural complications include perforation (in 4 patients), tamponade requiring pericardiocentesis (in 1 patient) and periprocedural myocardial infarction (in 1 patient). The LAST technique is similar to “mini-STAR”, however, instead of advancing a Fielder hydrophilic guidewire to re-enter the distal true lumen, a Confianza Pro 12 or Pilot 200 wire with an acute distal bend is used [27,37].
Retrograde approach: Retrograde approach for CTO was initially described by Kahn and Harzler in 1990 for performing balloon angioplasty of a left anterior descending artery CTO via a saphenous vein graft [38]. In some cases, CTO cannot be crossed with an antegrade approach but the true lumen can be penetrated from a retrograde direction (via epicardial or septal collateral vessel or a bypass graft that connects with the distal vessel). Unlike the antegrade approach, the guidewire is introduced directly into the distal vessel and advanced to approach the end of the convex distal fibrous cap of CTO. As compared to proximal cap, distal cap is usually less resistant which makes penetration easier. The retrograde wire can either serve as a marker or create a channel to facilitate passage of a second wire in an antegrade direction. Retrograde approach is useful for true ostial left descending artery occlusions without a stump, or medial RCA occlusion with a large marginal branch arising at the site of the CTO. It can be performed by three routes: septal collaterals, epicardial collaterals and bypass grafts. Among these, septal collaterals (owing to less tortuosity) are commonly used [39]. Epicardial collaterals are used in rare cases as they are more prone to rupture upon dilatation as well as serious clinical consequences of the complication. Euro CTO club consensus recommend Werner classification (for the assessment of collateral connections grade) to determine tortuosity and continuity of collaterals [3].
a. Retrograde true lumen puncture: It is considered as the most “pure” form of the retrograde technique. After crossing of collateral, the hydrophilic guidewire is advanced to CTO in a retrograde manner, followed by advancement of the microcatheter or OTW balloon. The CTO is then crossed in a retrograde manner, either using the same guidewire or a stiffer guidewire. Inflation of retrograde balloon, antegrade IVUS or use of stiffer, tapered tip or hydrophilic wires facilitates crossing of CTO in a retrograde manner. Use of antegrade IVUS is also proved useful for directing the retrograde guidewire into the proximal true lumen and for confirmation of intraluminal position of the wire to avoid dissection of the proximal vessel if the wire crossed subintimally [40,41].
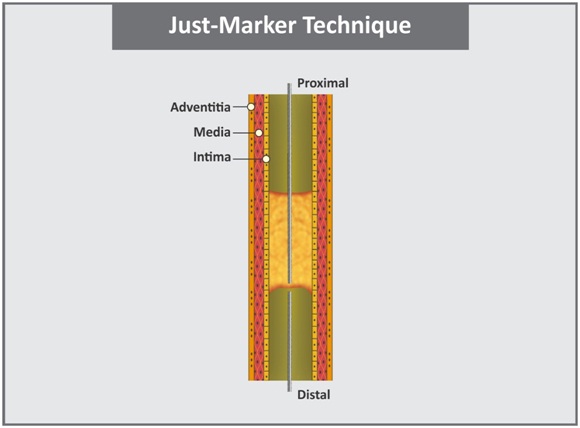
b. Just-marker technique: It is relatively a simple form of retrograde strategies. After crossing collateral, the retrograde wire is advanced to the distal cap of the lesion [Table/Fig-7]. The retrograde wire acts as a marker of the distal true lumen and serves as a target for antegrade wire (without attempting to penetrate occlusion lesion in a retrograde manner). This allows continuous visualization of the distal true lumen location and reduces the use of contrast [42,43].
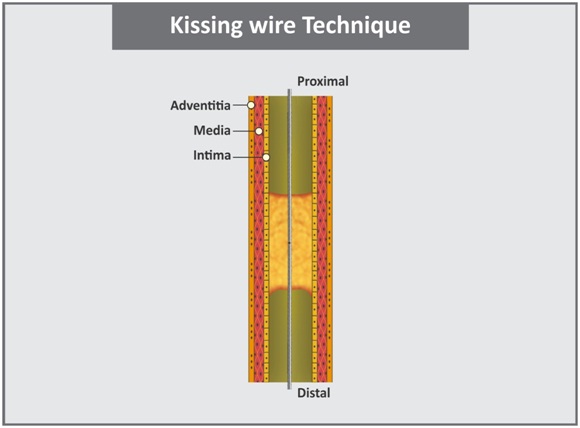
c. Kissing wire technique: It is similar to the Just-marker technique. Here, the retrograde wire is advanced to the proximal end of the lesion [Table/Fig-8]. The antegrade wire is also advanced to lesion until the ends of the wires meet and then the antegrade wire follows the path made by the retrograde wire in the true lumen in the distal vessel [42].
d. CART technique: It was first described by Surmely for retrograde penetration and dilatation of the lesion, thus creating a large subintimal space which communicates with the true lumen [44]. In this technique, an antegrade wire is advanced from the proximal true lumen into the subintimal space (at CTO) followed by insertion of retrograde wire into distal true lumen and then into the CTO subintimal space [Table/Fig-9]. Here, the retrograde balloon advancement through a collateral vessel might require multiple low pressure small balloon inflations for septal collateral vessel dilation [45].
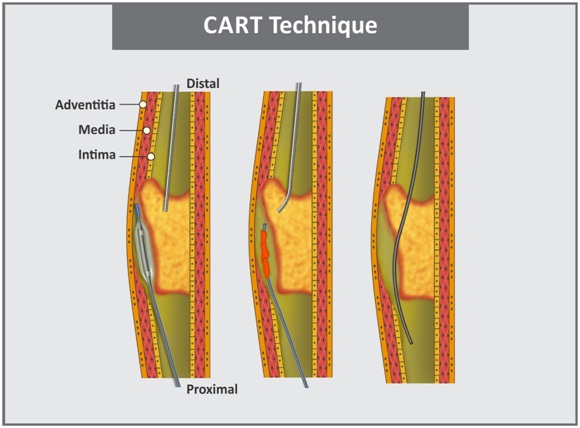
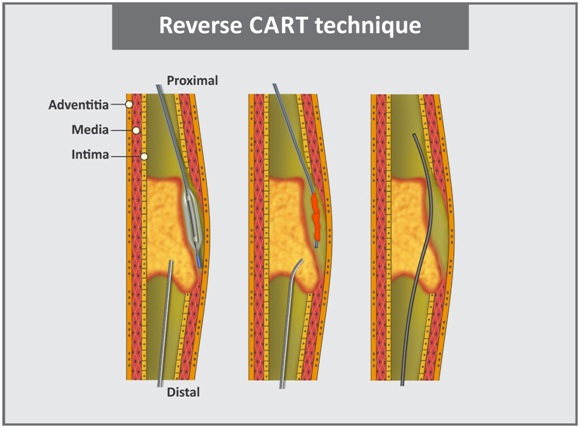
e. Reverse CART technique: It is similar to the CART technique except for that a balloon is advanced over the antegrade guidewire to the proximal part of the occlusion and the retrograde wire crosses into the proximal true lumen [45]. This technique [Table/Fig-10] allows for the use of IVUS to guide crossing of retrograde guidewire into proximal true lumen. However, long course and many angulations need to be encountered while performing this technique [46].
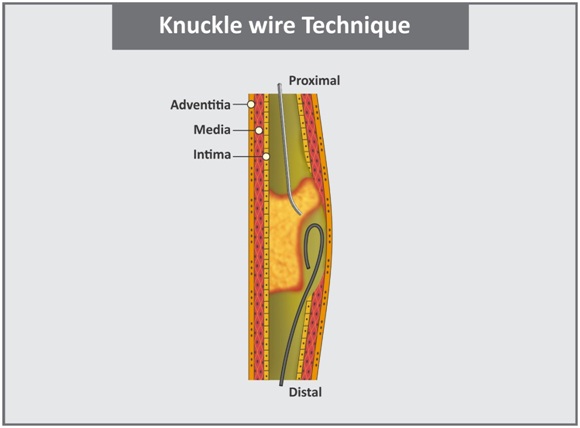
f. Knuckle wire technique: It is preferred for long segments of occlusion which involves use of a loop in the retrograde wire to create a dissection plane [Table/Fig-11]. The loop is advanced within the sub-intimal space without causing perforation. The principle is similar to the STAR procedure [39].
Balloon Uncrossable CTOs
In certain cases, the balloons are not able to cross the lesion even though the guidewire cross the lesions. Such CTOs are termed as “Balloon Uncrossable” CTOs leading to the failure of PCI in 2%–9% of CTO. The major reasons could be coronary tortuosity or heavy calcification within the CTO lesions [47,48]. Few newly developed techniques for balloon uncrossable CTOs are described below.
a. Wire-cutting technique: In this technique, two guidewires are inserted into the distal true lumen, followed by advancing a balloon over the guidewire to the proximal cap of the CTO. The balloon is then inflated which produces crushing effect (by pressing second guidewire between the balloon and the proximal cap). Inflation of balloon is followed by rapid withdrawal of second guidewire, which facilitates balloon crossing. The limitation of this technique is to pass two guidewires into the distal true lumen. This technique was first reported by Hu et al. with procedural success rate of 62.5% [49].
b. Seesaw balloon-wire cutting technique: This novel technique was described by Yue Li et al., which involved easy operation, low cost, and decreased fluoroscopy time [51]. The procedure is described as follows: a guidewire (guidewire A) is first inserted into the distal true lumen of CTOs followed by insertion of another stiffer hydrophilic guidewire (guidewire B) along with guidewire A. Once the guidewires are inserted into distal true lumen, two short and low-profile balloons are advanced over the two guidewires i.e., balloon A is first advanced over guidewire A as distally as possible and inflated with high pressure (≥18atm) to press guidewire B which produces a cutting power to crush the proximal fibrous cap of the CTOs. Subsequently, balloon A is withdrawn slightly and balloon B is advanced over guidewire B as distally as possible and inflated to press guidewire A (for similar cutting effect). The procedure is repeated until one of them cross the occluded segment. Here, one balloon provides extra back-up support and facilitate the other balloon to pass through the CTO lesion. The authors have reported procedural success rate of 81% and no incidence of serious complication. However, the heavy circular calcification within the CTO (balloon-uncrossable) lesions may not be treated successfully by this technique. Another limitation of this technique is insertion of second guidewire [50].
c. Subintimal distal anchor: Michael et al., described this technique after successfully treating an elderly patient by this novel technique [50]. The technique involves advancement of second coronary guidewire through the subintimal space distal to the occlusion site with a balloon, followed by inflation of the balloon to anchor the first guidewire that has already crossed distal true lumen of CTO. The anchoring of the guidewire facilitates balloon crossing on the initial wire. The limitation of this technique is the requirement of subintimal crossing which may not be always feasible or it may compress distal true lumen due to formation of subintimal hematoma [51].
Special Devices for Refractory Revascularization of Chronic Total Occlusion
Numerous devices, such as Magnum/Magnarail system, Kensey catheter, ROTACS low speed rotational atherectomy catheter and Excimer Laser Wire have been developed for the successful PCI of CTO [52]. The devices for successful CTO recanalization must have three characteristics:steerability to choose the best path, ability to differentiate potential lumen and vessel wall before perforation or dissection occurs, and strength (force or energy) to move forward in fibrotic/calcified CTO.
1. Safe cross-RF total occlusion crossing system: The Safe cross-RF radio frequency total occlusion crossing system (Intra Luminal Therapeutics, Carlsbad, California) is designed to ablate total coronary occlusions using radiofrequency energy which could not be treated using conventional guidewires [47,53]. The device emits near infrared radiation (100 ms pulses and 250-500 kHz) to the tip of 0.014 inch intermediate-stiffness guidewire. The emitted radiation energy can ablate the dense fibrotic occlusions and thereby facilitate advancement of the guidewire within the occluded segment. Safe-Cross system also implements optical coherence reflectometry, a new guidance system, which uses low coherence light transmitted from 0.007 inch optical fibre incorporated into the tip of the device. The difference between plaque and artery wall is measured by absorption and scatter pattern of the reflected radiation and displayed in a waveform. Thus, this visible and audible signal alerts the operator if the wire gets too close to arterial tissue and thereby preventing perforation. Additionally, when proximity of the vessel wall is not detected, under these circumstances only radiofrequency pulses can be activated. Guided Radio Frequency Energy Ablation of Total Occlusions Registry Study (GREAT-Registry) demonstrated device success in 63 of 116 patients (54.3%) and major adverse events occurred in 6.9% (which predominantly consisted of isolated increases in cardiac enzymes with no procedure-related deaths, Q-wave myocardial infarctions, or emergency bypass operations) [54]. In this study, the perforation due to the device was observed in 1 patient (0.9%) though clinical perforation occurred in 2.6% of patients.
2. Frontrunner catheter: Frontrunner-XP catheter is a low profile guiding catheter (0.039") with actuating jaws that open to 2.3 mm [47,53]. A 4.5F micro guide catheter provides support for the advancement and retraction. The steerability and maneuverability of the device has been because of its effective torque control and shapeable distal tip. The device has hydrophilic coating along the entire catheter length to facilitate CTO crossing. The currently available device can angle to 25 and 36 and thereby navigate tortuous vessels (It should be noted that the device has undergone several iterations to reduce its profile and enhance crossing ability and torque response). The device incorporates bioptomes like jaws at hinged distal tip by which it creates and propagates an intraluminal pathway in CTO using controlled blunt microdissection. As there is different elastase between intraluminal plaque and adventitia, blunt dissection preferentially disrupts the atherosclerotic plaque without affecting the integrity of the outer arterial wall layer. Orlicet et al., evaluated efficacy and safety of recanalization using the Frontrunner coronary catheter in 50 patients (with 50 refractory CTOs) [55]. The device success was obtained in 25 occlusions (50%). However, serious adverse events occurred in five (10%) patients within 30-day follow-up.
3. Crosser System: The CROSSER coronary catheter (Flow Cardia, Sunnnyvale, California) is compatible with 6 Fr guide catheter and 0.0014 inch guidewire and has a blunt stainless steel tip. The system consists of a generator, transducer and single use catheter. A generator of the system converts alternating current line power into high frequency current which is then transferred to piezoelectric crystals within the transducer. As a result of current, piezoelectric crystals expand and contract. This vibrational energy is propagated to the stainless steel tip of the device. The device mechanically vibrates against the face of the CTO at 20kHz (high frequency vibrations) at a stroke depth of approximately 20μm. The mechanical vibrations produced at the tip change hardness and structure of the plaque and thereby facilitate propagation through CTO. Apart from the mechanical impact, the vibrations of the device also create vapour-filled microbubbles (in the blood and saline). As microbubbles expand and implode, they break the molecular bond and thereby erode the solid surface of CTO. In order to avoid local tissue heating (due to vibration of device), continuous saline irrigation at a distal tip is delivered to maintain temperature. Melzi et al., treated 28 patients (30 refractory CTOs) and reported success rate of 63% with 7.1% thirty-day major adverse cardiac event [56]. US FACTOR was a prospective, non-randomized and multicentre study which evaluated safety and feasibility of CROSSER system in 150 refractory CTOs in 125 patients [57]. The success rate of the study was 61% and 30-day major adverse cardiac event rate was 8.8%. However, Galassi et al., treated 46 complex CTO lesions (in 45 patients) with CROSSER system, used as a primary strategy. The median occlusion duration was 15 months and severe calcification was noted in 93.5% cases. The authors reported clinical success of CROSSER system in 84.8% cases. There was no peri-procedural perforation, myocardial infarction or 30-day major adverse cardiac event [58].
Conclusion
PCI of CTO should be considered as a preferred management modality for patients in whom a high procedural success is anticipated. As described in this review, the tremendous progress in guidewire technology and techniques coupled with dedicated devices for refractory CTOs has increased success rate of percutaneous revascularization of the lesions. This advancement in techniques and devices, specifically designed to cross totally occluded vessels, have provided us a potential to achieve complete revascularisation in CTO lesions. However, spending time for performing the procedure and gaining experience with the techniques and devices specifically designed for refractory CTOs is equally important for the safe and successful recanalization. Ultimately, adequate knowledge of contemporary techniques/devices would help the interventional cardiologists to overcome formidable challenges in increasingly complex cases of CTO and to deliver safe and effective percutaneous treatment to patients.
Abbreviations
CTO: Chronic total occlusion; EBU: Extra-backup type catheter; IVUS: Intravascular ultrasound;OTW: Over the wire; PCI: Percutaneous coronary intervention; PTFE: Polytetrafluoroethylene; RCA: Right coronary artery; STAR: Subintimal tracking and re-entry; TIMI: Thrombolysis in myocardial infarction.
[1]. Ambrose JA, Singh M, Pathophysiology of coronary artery disease leading to acute coronary syndromesF1000 Prime Rep 2015 7:08 [Google Scholar]
[2]. Shah PB, Management of coronary chronic total occlusionCirculation 2011 123:1780-84. [Google Scholar]
[3]. Sianos G, Werner GS, Galassi AR, Papafaklis MI, Escaned J, Hildick-Smith D, Recanalisation of chronic total coronary occlusions: 2012 consensus document from the Euro CTO clubEuro Intervention 2012 8:139-45. [Google Scholar]
[4]. Kahn JK, Angiographic suitability for catheter revascularization of total coronary occlusions in patients from a community hospital settingAm Heart J 1993 126:561-64. [Google Scholar]
[5]. Anderson HV, Shaw RE, Brindis RG, Hewitt K, Krone RJ, Block PC, A contemporary overview of percutaneous coronary interventions. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR)J Am Coll Cardiol 2002 39:1096-103. [Google Scholar]
[6]. Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, Percutaneous coronary intervention in the current era compared with 1985-1986: the National Heart, Lung, and Blood Institute RegistriesCirculation 2000 102:2945-51. [Google Scholar]
[7]. Srinivas VS, Brooks MM, Detre KM, King SB 3rd, Jacobs AK, Johnston J, Contemporary percutaneous coronary intervention versus balloon angioplasty for multivessel coronary artery disease: a comparison of the National Heart, Lung and Blood Institute Dynamic Registry and the Bypass Angioplasty Revascularization Investigation (BARI) studyCirculation 2002 106:1627-33. [Google Scholar]
[8]. Hoye A, van Domburg RT, Sonnenschein K, Serruys PW, Percutaneous coronary intervention for chronic total occlusions: the Thorax center experience 1992-2002Eur Heart J 2005 26:2630-36. [Google Scholar]
[9]. Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, Salemme L, Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE)J Am Coll Cardiol 2003 41:1672-78. [Google Scholar]
[10]. Suero JA, Marso SP, Jones PG, Laster SB, Huber KC, Giorgi LV, Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experienceJ Am Coll Cardiol 2001 38:409-14. [Google Scholar]
[11]. Baks T, van Geuns RJ, Duncker DJ, Cademartiri F, Mollet NR, Krestin GP, Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusionsJ Am Coll Cardiol 2006 47:721-25. [Google Scholar]
[12]. Kirschbaum SW, Baks T, van den Ent M, Sianos G, Krestin GP, Serruys PW, Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusionsAm J Cardiol 2008 101:179-85. [Google Scholar]
[13]. Serruys PW, Umans V, Heyndrickx GR, van den Brand M, de Feyter PJ, Wijns W, Elective PTCA of totally occluded coronary arteries not associated with acute myocardial infarction; short-term and long-term resultsEur Heart J 1985 6:2-12. [Google Scholar]
[14]. Kereiakes DJ, Selmon MR, McAuley BJ, McAuley DB, Sheehan DJ, Simpson JB, Angioplasty in total coronary artery occlusion: experience in 76 consecutive patientsJ Am Coll Cardiol 1985 6:526-33. [Google Scholar]
[15]. Maiello L, Colombo A, Gianrossi R, Mutinelli MR, Bouzon R, Thomas J, Coronary angioplasty of chronic occlusions: factors predictive of procedural successAm Heart J 1992 124:581-84. [Google Scholar]
[16]. Stewart JT, Denne L, Bowker TJ, Mulcahy DA, Williams MG, Buller NP, Percutaneous transluminal coronary angioplasty in chronic coronary artery occlusionJ Am Coll Cardiol 1993 21:1371-76. [Google Scholar]
[17]. Galassi AR, Tomasello SD, Reifart N, Werner GS, Sianos G, Bonnier H, In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registryEuro Intervention 2011 7:472-79. [Google Scholar]
[18]. Thompson CA, Jayne JE, Robb JF, Friedman BJ, Kaplan AV, Hettleman BD, Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experienceJACC Cardiovasc Interv 2009 2:834-42. [Google Scholar]
[19]. Rinfret S, Joyal D, Nguyen CM, Bagur R, Hui W, Leung R, Retrograde recanalization of chronic total occlusions from the transradial approach; early Canadian experienceCatheter Cardiovasc Interv 2011 78:366-74. [Google Scholar]
[20]. Timmis AD, Percutaneous transluminal coronary angioplasty: catheter technology and procedural guidelinesBr Heart J 1990 64:32-35. [Google Scholar]
[21]. Giubilato S, Galassi AR, Tomasello SD, Percutaneous. Recanalization of Chronic Total Occlusion (CTO) Coronary Arteries: Looking Back and Moving Forward 2013 INTECH Open Access Publisher [Google Scholar]
[22]. Ge JB, Current status of percutaneous coronary intervention of chronic total occlusionJ Zhejiang Univ Sci B 2012 13:589-602. [Google Scholar]
[23]. Brilakis ES, Grantham JA, Rinfret S, Wyman RM, Burke MN, Karmpaliotis D, A percutaneous treatment algorithm for crossing coronary chronic total occlusionsJACC Cardiovasc Interv 2012 5:367-79. [Google Scholar]
[24]. Manica JL, Piazza L, Butera G, The use of a wire control catheter to treat complex pulmonary artery or vein anatomyJ Invasive Cardiol 2012 24:E148-52. [Google Scholar]
[25]. Godino C, Sharp AS, Carlino M, Colombo A, Crossing CTOs-the tips, tricks, and specialist kit that can mean the difference between success and failureCatheter Cardiovasc Interv 2009 74:1019-46. [Google Scholar]
[26]. Sakes A, Regar E, Dankelman J, Breedveld P, Crossing total occlusions: Navigating towards recanalizationCardiovasc Eng Technol 2016 7:103-17. [Google Scholar]
[27]. Murarka S, Lassetter JE, Waters KL, Scherger S, Heuser RR, Chronic total occlusions in the coronary vasculatureCath Lab Digest 2010 18:10 [Google Scholar]
[28]. Mishra S, Language of CTO interventions–Focus on hardwareIndian Heart J 2016 http://dx.doi.org/ 10.1016/j.ihj.2016.06.015 [Google Scholar]
[29]. Wong GB, Price MJ, Teirstein PS, Guidewire techniques and technologies: hydrophilic versus stiff wire selectionHandbook of Chronic Total Occlusions 2007 :11 [Google Scholar]
[30]. Carlino M, Latib A, Godino C, Cosgrave J, Colombo A, CTO recanalization by intraocclusion injection of contrast: the microchannel techniqueCatheter Cardiovasc Interv 2008 71:20-26. [Google Scholar]
[31]. Srivatsa SS, Edwards WD, Boos CM, Grill DE, Sangiorgi GM, Garratt KN, Histologic correlates of angiographic chronic total coronary artery occlusions: influence of occlusion duration on neovascular channel patterns and intimal plaque compositionJ Am Coll Cardiol 1997 29:955-63. [Google Scholar]
[32]. Stone GW, Kandzari DE, Mehran R, Colombo A, Schwartz RS, Bailey S, Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part ICirculation 2005 112:2364-72. [Google Scholar]
[33]. Strauss BH, Segev A, Wright GA, Qiang B, Munce N, Anderson KJ, Microvessels in chronic total occlusions: pathways for successful guidewire crossing?J Interv Cardiol 2005 18:425-36. [Google Scholar]
[34]. Colombo A, Mikhail GW, Michev I, Iakovou I, Airoldi F, Chieffo A, Treating chronic total occlusions using subintimal tracking and reentry: the STAR techniqueCatheter Cardiovasc Interv 2005 64:407-11. [Google Scholar]
[35]. Carlino M, Godino C, Latib A, Moses JW, Colombo A, Subintimal tracking and re-entry technique with contrast guidance: a safer approachCatheter Cardiovasc Interv 2008 72:790-96. [Google Scholar]
[36]. Galassi AR, Tomasello SD, Costanzo L, Campisano MB, Barrano G, Ueno M, Mini-STAR as bail-out strategy for percutaneous coronary intervention of chronic total occlusionCatheter Cardiovasc Interv 2012 79:30-40. [Google Scholar]
[37]. Michael TT, Papayannis AC, Banerjee S, Brilakis ES, Subintimal dissection/reentry strategies in coronary chronic total occlusion interventionsCirc Cardiovasc Interv 2012 5:729-38. [Google Scholar]
[38]. Kahn JK, Hartzler GO, Retrograde coronary angioplasty of isolated arterial segments through saphenous vein bypass graftsCathet Cardiovasc Diagn 1990 20:88-93. [Google Scholar]
[39]. Brilakis ES, Grantham JA, Thompson CA, DeMartini TJ, Prasad A, Sandhu GS, The retrograde approach to coronary artery chronic total occlusions: a practical approachCatheter Cardiovasc Interv 2012 79:3-19. [Google Scholar]
[40]. Furuichi S, Satoh T, Intravascular ultrasound-guided retrograde wiring for chronic total occlusionCatheter Cardiovasc Interv 2010 75:214-21. [Google Scholar]
[41]. Wu EB, Chan WW, Yu CM, Antegrade balloon transit of retrograde wire to bail out dissected left main during retrograde chronic total occlusion intervention-a variant of the reverse CART techniqueJ Invasive Cardiol 2009 21:e113-18. [Google Scholar]
[42]. Saito S, Different strategies of retrograde approach in coronary angioplasty for chronic total occlusionCatheter Cardiovasc Interv 2008 71:8-19. [Google Scholar]
[43]. Niccoli G, Ochiai M, Mazzari MA, A complex case of right coronary artery chronic total occlusion treated by a successful multi-step Japanese approachJ Invasive Cardiol 2006 18:E230-33. [Google Scholar]
[44]. Surmely JF, Tsuchikane E, Katoh O, Nishida Y, Nakayama M, Nakamura S, New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART techniqueJ Invasive Cardiol 2006 18:334-38. [Google Scholar]
[45]. Michael TT, Papayannis AC, Banerjee S, Brilakis ES, Subintimal dissection/reentry strategies in coronary chronic total occlusion interventionsCirculation: Cardiovasc Interv 2012 5:729-38. [Google Scholar]
[46]. Rathore S, Katoh O, Tuschikane E, Oida A, Suzuki T, Takase S, A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde trackingJACC Cardiovasc Interv 2010 3:155-64. [Google Scholar]
[47]. Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part IICirculation 2005 112:2530-37. [Google Scholar]
[48]. Pagnotta P, Briguori C, Mango R, Visconti G, Focaccio A, Belli G, Rotational atherectomy in resistant chronic total occlusionsCatheter Cardiovasc Interv 2010 76:366-71. [Google Scholar]
[49]. Hu XQ, Tang L, Zhou SH, Fang ZF, Shen XQ, A novel approach to facilitating balloon crossing chronic total occlusions: the "wire-cutting" techniqueJ Interv Cardiol 2012 25:297-303. [Google Scholar]
[50]. Li Y, Li J, Sheng L, Gong Y, Li W, Sun D, "Seesaw balloon-wire cutting" technique as a novel approach to "balloon-uncrossable" chronic total occlusionsJ Invasive Cardiol 2014 26:167-70. [Google Scholar]
[51]. Michael TT, Banerjee S, Brilakis ES, Subintimal distal anchor technique for "balloon-uncrossable" chronic total occlusionsJ Invasive Cardiol 2013 25:552-54. [Google Scholar]
[52]. Waksman R, Saito S, Chronic total occlusions: a guide to recanalization 2013 John Wiley & Sons [Google Scholar]
[53]. Stone GW, Colombo A, Teirstein PS, Moses JW, Leon MB, Reifart NJ, Percutaneous recanalization of chronically occluded coronary arteries: procedural techniques, devices, and resultsCatheter Cardiovasc Interv 2005 66:217-36. [Google Scholar]
[54]. Baim DS, Braden G, Heuser R, Popma JJ, Cutlip DE, Massaro JM, Utility of the Safe-cross-guided radiofrequency total occlusion crossing system in chronic coronary total occlusions (results from the Guided Radio Frequency Energy Ablation of Total Occlusions Registry Study)Am J Cardiol 2004 94:853-58. [Google Scholar]
[55]. Orlic D, Stankovic G, Sangiorgi G, Airoldi F, Chieffo A, Michev I, Preliminary experience with the frontrunner coronary catheter: novel device dedicated to mechanical revascularization of chronic total occlusionsCatheter Cardiovasc Interv 2005 64:146-52. [Google Scholar]
[56]. Melzi G, Cosgrave J, Biondi-Zoccai GL, Airoldi F, Michev I, Chieffo A, A novel approach to chronic total occlusions: the crosser systemCatheter Cardiovasc Interv 2006 68:29-35. [Google Scholar]
[57]. Tiroch K, Cannon L, Reisman M, Caputo R, Caulfield T, Heuser R, High-frequency vibration for the recanalization of guidewire refractory chronic total coronary occlusionsCatheter Cardiovasc Interv 2008 72:771-80. [Google Scholar]
[58]. Galassi AR, Tomasello SD, Costanzo L, Campisano MB, Marza F, Tamburino C, Recanalization of complex coronary chronic total occlusions using high-frequency vibrational energy CROSSER catheter as first-line therapy: a single center experienceJ Interv Cardiol 2010 23:130-38. [Google Scholar]