Leptin is a hormone produced mainly by the adipose tissue and plays roles in body weight homeostasis, neuroendocrine function, fertility, immune function and angiogenesis [1]. Leptin acts on the hypothalamic receptor called Obesity Gene Receptor (OBR) where it exerts the control of food intake and body weight through a negative feedback mechanism (anorexigenic function) [2]. Leptin has been thought to be expressed and secreted by adipocytes, however other tissues have been identified to secrete leptin such as stomach [3], salivary glands [4], placenta [5], mammary epithelial cells [6] and lung [7]. Leptin secretion is positively correlated with body mass and therefore, obesity is accompanied by high circulating leptin levels [8].
Leptin upregulates Cyclin Dependent Kinase-2 (CDK2) and cyclin D1 levels as well as inactivates the cell cycle inhibitor, pRb and consequently induces cell cycle progression [9]. Additionally, leptin can regulate apoptosis; induce migration and expression of matrix degrading enzymes and angiogenic factors such as Vascular Endothelial Growth Factor (VEGF) [10]. Increased proliferation, deregulated apoptosis and promotion of angiogenesis are important events in the process of carcinogenesis.
Obesity in adults is associated with increased risk of cardiovascular disease, diabetes, some forms of cancer, and numerous other health disorders [11]. Many epidemiological studies have found a link between excess adiposity and malignant melanoma (MM) [12,13]. However, others have denied this link [14]. Obesity has been found to be associated with non-melanoma skin cancers according to Canadian study [15], whereas an absence of association with basal cell carcinoma has been demonstrated in Australian study [16]. It may be that obesity is a more relevant risk factor for skin cancer in areas of lower ultraviolet radiation (UVR) exposure [15].
Therefore, the current study tried to assess leptin localization and expression in non melanoma skin cancer to verify its possible role in pathogenesis of this cancer and if obesity could be blamed in increased skin cancer risk.
Materials and Methods
This study was carried out on 27 patients (13 BCC and 14 SCC) presented with skin malignant ulcer in Outpatient Clinic, Dermatology Department, Faculty of Medicine, Menoufia University in the period between January, 2014 and July 2015. Nineteen healthy volunteers were included in the current study as a control group. The study was approved by Committee of Human Rights in Research of Menoufia University. An informed consent was signed by all participants.
Each patient in the study was subjected to the following:
Complete history taking and full clinical assessment including general and dermatologic examination;
The suspected ulcer was evaluated regarding its site, size, edge, base and floor, in addition to assessment of regional lymph nodes;
Excision biopsy with safety margins of the clinically evaluated ulcers were performed in Surgery Department;
The biopsies were sent to Pathology Department for routine tissue processing with paraffin embedded blocks formation. Several sections were cut from each block, one to be stained by haematoxylin and eosin staining for diagnosis, evaluation of the margins, number of mitotic figures, and variant in BCC together with grade and stage in SCC.
Immunohistochemical Staining of Leptin
The method used for immunostaining was streptavidin-biotin–amplified system. From each block, 4μm thick sections were cut on positive charged slides, which were subjected to subsequent steps of deparaffinization and rehydration. Antigen retrieval was performed by boiling in citrate buffer saline (pH 6) followed by cooling at room temperature. The primary antibody used was rabbit polyclonal antihuman leptin (concentrated, Santa Cruz Biotechnology Inc., Heidelberg, Germany), diluted in antibody diluent (1:200) and incubated overnight at room temperature. Then the secondary antibody (Ultravision detection system anti-polyvalent Horseradish Peroxidase (HRP)/3,3’-Diaminobenzidine (DAB), ready-to-use, Neomarker) was applied with DAB as a chromogenic substrate and Mayer’s haematoxylin as a counter stain. Blood vessels (built-in internal control) were used as positive control. Replacement of the primary antibody step with a blocking buffer was included in the staining procedure as a negative control.
Interpretation of Leptin Immunostaining
Cytoplasmic immunoreactivity in any number of cells was required to assign leptin positivity. Leptin expression was assessed in tumour cells and the surrounding stroma. The percentage of immunoreactivity in tumour cells was evaluated and expressed as a mean, median and range.
Assessment of Microvessel Density (MVD)
Immunohistochemical staining for CD34 (monoclonal mouse antibody, ready to use, Neomarker, Labvision, USA) was used to highlight the vessels for calculation of MVD. Areas of highest vascularity designated as hot spots were selected for counting. Vessels were counted in 10 high power fields the average was considered as MVD.
Statistical Analysis
Data were collected, tabulated, and statistically analysed using a personal computer with the “Statistical Package for the Social Sciences” (SPSS) version 20.0. The Chi-square and the Fisher-exact tests were used for comparisons between qualitative variables. Mann-Whitney (U) was used for comparisons between quantitative variables. Spearman’s correlation coefficient (r) was used to assess the linear correlation between two quantitative variants. p < 0.05 was considered the cut-off value for significance(s) and p < 0.01 was considered as highly significant.
Results
The clinical and pathological features of BCC and SCC are presented in [Table/Fig-1].
The clinical and pathological data of BCC and SCC cases.
| Parameters | | BCC | SCC |
|---|
| Age/year | mean ± SD | 58.6 ± 14.1 | 52.6 ± 20.1 |
| median | 60 | 56 |
| range | 26 - 80 | 12 - 80 |
| Sex | male | 6 (46.2%) | 10 (71.4%) |
| female | 7 (53.8%) | 4 (28.6%) |
| M:F ratio | 1 : 1.2 | 1 : 0.4 |
| Size/cm | mean ± SD | 1.8 ± 0.8 | 4 ± 3 |
| median | 1.5 | 2.5 |
| range | 1 - 3.5 | 1 - 10 |
| Site | Head & Neck | 13 (100%) | 8 (57.1%) |
| Others | 0 (0%) | 6 (42.9%) |
| Type | Classic | 12 (92.3%) | |
| Adenoid | 1 (7.7%) |
| Margin | Negative | 8 (61.5%) | 12 (85.7%) |
| Positive | 5 (38.5%) | 2 (14.3%) |
| MVD | mean ± SD | 3.1 ± 2.1 | 17 ± 8.6 |
| median | 3 | 19 |
| range | 0 - 8 | 6 - 28 |
| Mitoses | mean ± SD | 2.9 ± 1.7 | 5.3 ± 4.5 |
| median | 2 | 4 |
| range | 1 - 7 | 1 - 14 |
| Grade | 1 | | 10 (71.4%) |
| 2 | 4 (28.6%) |
| 3 | 0 (0%) |
| T Stage | T1 | | 4 (28.6%) |
| T2 | 4 (28.6%) |
| T3 | 4 (28.6%) |
| T4 | 2 (14.3%) |
MVD, microvessel density
Leptin Expression in Normal Skin
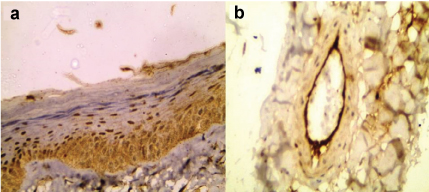
Leptin showed epidermal expression in 10 out of 19 cases (52.6%) [Table/Fig-2] with cytoplasmic pattern in three cases and both cytoplasmic and nuclear in seven cases. The expression was confined in basal and suprabasal layers. Endothelial cells [Table/Fig-2] and fibroblasts of dermis also showed leptin expression.
Cytoplasmic and nuclear expression of leptin in normal epidermis mainly in basal layer with scattered immunoreactivity in suprabasal layers (a). Prominent leptin expression in endothelial cells (b) (immunohistochemical staining 200X for (a) and 400X for (b).
Leptin Expression in BCC
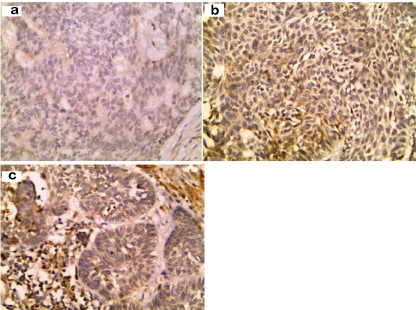
Only two (15.4%) BCC cases showed cytoplasmic leptin expression [Table/Fig-3] with a percentage ranging between 10% and 20%. Leptin was also expressed in stroma of five BCC cases localized mainly to inflammatory cells (38.5%) [Table/Fig-3]. There was no significant correlation between leptin expression by stromal cells in BCC and the studied parameters.
Absence of leptin expression in BCC (a). Cytoplasmic expression of leptin in tumour cells (b) and stromal inflammatory cells (c) of BCC (immunohistochemical staining 400X)
Leptin Expression in SCC
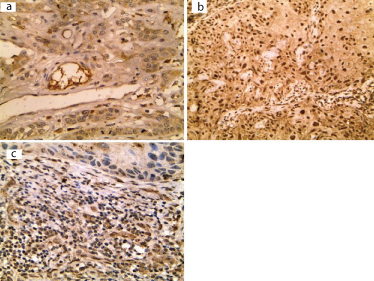
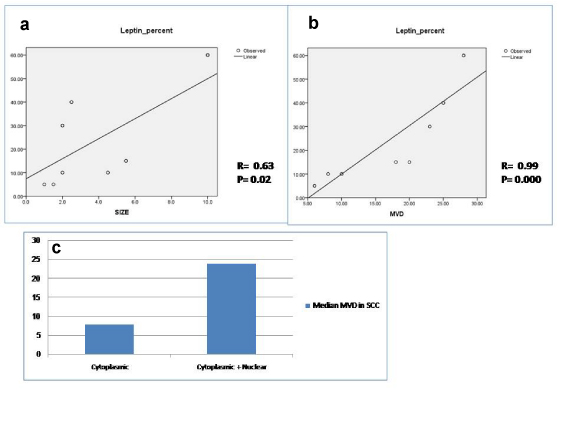
All cases of SCC showed leptin expression with cytoplasmic pattern in six cases (42.9%) [Table/Fig-4] and both cytoplasmic and nuclear expression in 8 cases (57.1%) [Table/Fig-4]. The percentage of expression ranged between 5% and 60% with a mean of 24.3 ± 15 and a median of 15. Eight cases showed stromal expression of leptin (57.1%). Leptin percentage of expression by tumour cells was positively correlated with tumour size (p=0.02) [Table/Fig-5] and microvessel density (p=0.000) [Table/Fig-5]. High MVD was significantly associated with cytoplasmic pattern compared to cases showing both cytoplasmic and nuclear pattern of leptin expression (P= 0.001) [Table/Fig-5].
Cytoplasmic (a) and nuclear (b) immunoreactivity of leptin in tumour cells of SCC. Leptin expression in inflammatory stroma of SCC (immunohistochemical staining 400X)
Positive correlation between percentage of leptin expression and both tumour size (a) and microvessel density in SCC (b). Microvessel density is higher in SCC showing cytoplasmic and nuclear expression compared to cases showing cytoplasmic expression only.
Leptin expression by inflammatory cells of the stroma of SCC was significantly associated with old age (p=0.04), large tumour size (p=0.02), tumours arising in sites other than head and neck (p=0.01) and with advanced stage (p=0.01) [Table/Fig-6].
The relationship between leptin expression by stromal cells and the clinicopathological parameters in SCC.
| No. of cases | Negative (6 cases) | Positive (8 cases) | Test | p-value |
|---|
| Age/year | mean ± SD | 14 | 40 ± 21.7 | 62 ± 13.2 | U = 8 | 0.04 S |
| median | 52 | 61.5 |
| range | 12 - 56 | 45 - 80 |
| Sex | male | 10 | 4 (40%) | 6 (60%) | FE = 0.11 | 1 |
| female | 4 | 2 (50%) | 2 (50%) |
| Size/cm | mean ± SD | 14 | 2.± 0.6 | 5.5 ± 3.1 | U = 6 | 0.02 S |
| median | 2 | 5 |
| range | 1 - 2.5 | 2 -10 |
| Site | H&N | 8 | 6 (75%) | 2 (25%) | FE = 7.9 | 0.01 S |
| others | 6 | 0 (0%) | 6 (100%) |
| Grade | well | 10 | 6 (60%) | 4 (40%) | FE= 4.2 | 0.09 |
| moderate | 4 | 0 (0%) | 4 (100%) |
| T Stage | 1 & 2 | 8 | 6 (75%) | 2 (25%) | FE = 7.9 | 0.01 S |
| 3 & 4 | 6 | 0 (0%) | 6 (100%) |
| Margin | negative | 12 | 6 (50%) | 6 (50%) | FE = 1.8 | 0.5 |
| positive | 2 | 0 (0%) | 2 (100%) |
| MVD | mean ± SD | 14 | 18 ± 9.3 | 16.3 ± 8.5 | U = 24 | 1 |
| median | 23 | 14 |
| range | 6 - 25 | 8 - 28 |
| Mitosis | mean ± SD | 14 | 3.3 ± 1.9 | 6.8 ± 5.4 | U = 16 | 0.3 |
| median | 4 | 5.5 |
| range | 1 - 5 | 2 - 14 |
MVD, microvessel density, H&N, head and neck.
Differences between Leptin Expression by Normal Epidermis and its Expression in BCC and SCC Separately
There was a marked down-regulation of leptin expression from normal epidermis to BCC (p=0.03) while its expression was up-regulated in SCC compared to normal epidermis (p=0.003) [Table/Fig-7].
Comparison between leptin expression by normal epidermis and its expression by tumour cells of BCC and SCC.
| Normal (19 cases) | BCC (13 cases) | SCC (14 cases) | Test | p | p1 | p2 |
|---|
| negative | 9 (47.4%) | 11 (84.6%) | 0 (0%) | X2= 19.8 | 0.000 HS ** | 0.03 | 0.003 |
| positive | 10 (52.6%) | 2 (15.4%) | 14 (100%) |
| Cytoplasmic | 3 (30%) | | 6 (42.9%) | FE= 0.4 | 0.5 | | |
| Nuclear + Cytoplasmic | 7 (70%) | 8 (57.1%) |
P tests the differences among normal, BCC and SCC, p1 tests the differences between normal and BCC, p2 tests the difference between normal and SCC
Comparison between BCC and SCC with Regard to Leptin Expression
Positive expression rate was significantly in favour of SCC, since all cases of SCC expressed leptin as compared to two cases of BCC (p=0.000). Furthermore, nuclear expression of leptin was significantly in favour of SCC compared to BCC, since the two positive cases of BCC showed only cytoplasmic localization (p=0.002). However, there were no significant differences between SCC and BCC considering leptin expression by stroma [Table/Fig-8].
Comparison between BCC and SCC with regard to leptin expression
| SCC | BCC | Test | p-value |
|---|
| Leptin in Tumour | Negative | 0 (0%) | 11 (84.6%) | FE= 19.9 | 0.000 HS |
| Positive | 14 (100%) | 2 (15.4%) |
| Cytoplasmic | 6 (42.9%) | 13 (100%) | FE= 10.6 | 0.002 HS |
| Cyto + Nuclear | 8 (57.1%) | 0 (0%) |
| Leptin in stroma | Negative | 6 (42.9%) | 8 (61.5%) | FE= 0.94 | 0.33 |
| Positive | 8 (57.1%) | 5 (38.5%) |
Discussion
The current study may be the first one to evaluate leptin expression in non melanoma skin cancer trying to verify if obesity could play a role in aetiopathogenesis of this common neoplasm or not.
The normal epidermis showed leptin expression in nearly half of cases in the keratinocytes of basal and suprabasal layers agreeing with Lin and Yang with cytoplasmic and nucleocytoplasmic patterns [17]. Nuclear pattern of leptin was also seen in epidermis of normal skin according to Seleit et al., 2015 [18].
Leptin is predominantly synthesized in adipocytes, including subcutaneous adipocytes [19]. However, synthesis of leptin and its receptors has also been detected in human and mice fibroblasts and keratinocytes [20,21]. Leptin and its full length receptor are present in the human epidermis at the gene and protein level confirmed by real-time quantitative PCR and immunohistochemistry [20,22,23].
Our study also showed leptin expression in endothelial cells and fibroblasts agreeing with Cao et al., [24]. Lower-intensity specific immunoreactivity for leptin is seen in endothelial cells, fibroblasts and adipocytes of the dermis. Local leptin synthesis and secraetion is strongly upregulated after skin injury [20] to promote wound healing by its angiogenic factor on endothelial cells [25] and its promoting role of keratinocyte proliferation and epithelialization as well as fibroblast proliferation and collagen synthesis [20,26,27].
The current study showed prominent leptin expression in all investigated cutaneous SCC compared to 78.8% of esophageal SCC [28] and 100% of laryngeal SCC [29]. The current study showed cytoplasmic pattern of leptin expression in 42.9% and nucleocytoplasmic expression in 57.1% of cases. Nuclear expression of leptin was reported in adenoid cystic carcinoma of lacrimal glands [30]
Large tumour size of SCC was associated with high percentage of leptin expression by tumour cells and with its expression in stroma. Tumour size is considered as a prognostic factor in SCC reflecting tumour bulk and determining T stage of the neoplasm. The association with size reflects the role of leptin in promoting the growth of neoplasms including cutaneous SCC by regulating cell cycle [9]. Furthermore, the percentage of leptin expression was positively correlated with microvessel density, reflecting its angiogenic role that is mediated through upregulation of vascular endothelial growth factor [10].
The stroma of malignant tumours is an active participant in the process of carcinogenesis since it secretes many cytokines and factors affecting tumour growth, metastasis and behaviour. The current study demonstrated leptin expression in the stroma of both SCC and BCC. The expression in the stroma of SCC was associated with large tumour size, advanced stage and with tumours arising in hidden areas other than head and neck. This means that stroma of SCC can lead to its progression by expression of leptin, which then promoted its size and worsened stage.
On the other hand, the expression of leptin in tumour cells of BCC was very limited (only 2 cases) according to our result, which may be explained by that the predisposing factor for BCC is mainly sun exposure and the role of obesity in this type of skin cancer is questioned. From the previous results, the role of leptin in pathogenesis and progression of SCC is obvious compared to BCC. This may be explained by that exposure to sun and ultraviolet rays are the most important risk factors for BCC while the risk factors for SCC are variable and not only confined to sun exposure. Signal Transducer and Activator of Transcription 3 (STAT3)-disrupted mice have a lower incidence of skin cancer, whereas transgenic animals with a constitutive active form of STAT3 may develop squamous cell carcinoma with a shorter latency [31], possibly due to the pro-proliferative response to leptin. The observation of alteration in the leptin pathway, leading to obesity, with an aberrant cytokine response to Ultra Violet Reactive (UVR) comes from animal experiments [32]. The compound effects of UVR and the alterations in the expression of adipocytokines associated with obesity, could contribute to cutaneous carcinogenesis [32]. This model might fit more with the observation linking obesity with cancer in general.
There is evidence that obesity-induced inflammation interacts with inflammation resulting from UVR favouring the process of skin carcinogenesis. Inflammatory mediators released secondary to UVB irradiation in mice have been found to be exacerbated in the skin of obese mice. For example, levels of the proinflammatory cytokines TNF-α, IL-6, and IL-1b were higher in the UVB exposed skin of obese mice suggesting a positive relationship between obesity and UVB-induced inflammation. Various inflammatory skin diseases have been developed on top of the higher levels of these proinflammatory cytokines [32], and sustained elevated levels of proinflammatory cytokines may predispose to increased skin cancer risk [33,34]. On the other hand, obesity is associated with a decreased risk of non-melanoma skin cancers according to Pothiawala et al., 2012 [35].
Regarding cutaneous melanoma, leptin was reported to be expressed in melanoma and nevi in high frequency while leptin receptor was higher in melanoma than in nevi [36]. Association has been consistently shown between adi- posity and increased risk of cancers of the endometrium, kidney, gallbladder (in women), breast (in postmeno-pausal women), and colon (particularly in men) [37].
Limitation
The most important limitation of the current study is absence of measurement of body mass index to divide the cases into obese and non obese and verify the role of obesity in induction of skin cancer.
Conclusion
Leptin could have a more important role in pathogenesis of cutaneous SCC rather than BCC, which may reflect the trivial role of obesity in induction of BCC. The expression of leptin by tumour and stromal cells of SCC could co-operate in its progression by promoting angiogenesis with subsequently acquiring large tumour size and then advanced stage.
MVD, microvessel density