Intra-ossseous periodontal vertical defects are at a higher risk of disease progression when left untreated [1]. These intra-bony lesions are not always readily accessible to periodontal debridement and hence may require access flap surgery alone or in association with bone-resective techniques or regenerative procedures [2]. Though periodontitis is initiated by bacterial colonization, host response plays an essential role in the breakdown of tissue and bone [3]. Hence, pharmacological agents, like bisphosphonates, which modulate host responses are being tested as treatment modalities for periodontal lesions [4–7].
Alendronate (ALN), an aminobisphosphonate is a potent inhibitor of osteoclast-mediated bone resorption with no adverse effect on the mineralization of bone [8,9]. The effectiveness of ALN in reducing alveolar bone resorption after mucoperiosteal flap surgery has been demonstrated in various studies [10–12]. A single local dose of ALN can give an adequate distribution of the bisphosphonate to the bone, because of the high affinity of bisphosphonates to calcium ions in the bone mineral [13,14]. The inhibition of resorptive activity is thought to produce a shift in bone turnover equilibrium to more osteoblastic activity [15]. Giuliani et al., demonstrated the potential of bisphosphonates in stimulating formation of osteoblast precursors in human bone marrow cultures and demonstrated early osteoblastogenesis in vivo in mice [16]. Bisphosphonates are potent in ameliorating the oxidative stress induced by periodontal infection [17]. Tenenbaum HC et al., in a state of the art review stated that, due to their potential in accelerating bone formation, bisphosphonates provide an interesting management strategy to stimulate osteogenesis in conjunction with regenerative materials around osseous defects [18].
Calcium Phosphates (CAPs) are efficient bone substitutes owing to their excellent biocompatibility and compositional similarity to bone mineral [19]. The intrinsic porosity of calcium phosphates allows for the incorporation of growth factors and drugs and their slow and sustained release over time [19,20]. Sustained release of ALN was observed over 21 days in an in vitro model of ALN loaded calcium phosphate cement [21]. β-TCP, is a calcium phosphate ceramic with a Ca/PO4 ratio similar to natural bone mineral. It has been used as a drug delivery vehicle in several studies [22–24].
ALN loaded bone substitutes have been developed in orthopedics for the treatment of osteoporosis [21,25]. This study was aimed to incorporate this concept of delivering ALN in a bone substitute for treating periodontal lesions. The rationale for using ALN was primarily for its potential as an inhibitor of bone resorption. However, its effect on bone formation in the treatment of human intra-osseous periodontal lesions has very limited documented research [26–31]. This study was designed as a randomized, active-controlled, parallel-arm study to evaluate the efficacy of 400μg ALN delivered in β-TCP, in inhibiting alveolar crestal resorption and enhancing bone formation in the treatment of periodontal intra-osseous defects.
Materials and Methods
Study participants: The present 6-month, randomized, prospective, parallel, interventional, active-controlled study included a total of 32 patients (15 males and 17 females) aged between 30 to 50 years, with chronic periodontitis and each having at least one inter-proximal periodontal defect. The patients were selected from the outpatient section of the Department of Periodontics, Panineeya Mahavidyalaya Institute of Dental Sciences and Research Centre, Hyderabad, India. Ethical clearance was obtained from the institutional ethics committee and written informed consent was obtained from all the participants.
Selection criteria: Systemically healthy patients having an intra-osseous defect with probing depth ≥ 7mm at baseline, presence of a ≥ 4mm vertical inter-proximal bone defect with at least one bony wall after surgical debridement and with no history of previous periodontal therapy were included in the study. Patients with known systemic diseases, aggressive periodontitis, smoking habit, known or suspected allergy to bisphosphonates, pregnant and lactating women, study tooth exhibiting tooth mobility greater than grade II and class III furcation defect were excluded from the study.
Study design and procedure: The patients were screened and eligible patients were randomly assigned by lottery method into the 400μg ALN + β-TCP + Saline (test) group and β-TCP + Saline (active-control) group in a ratio of 1:1. Only one periodontal defect per patient was included in the study. Investigator RK screened the patients and randomly assigned them to the test and control groups. Investigator RN performed the surgical procedure for all the study participants. Investigator JSP was blinded to the randomization and recorded the clinical soft tissue parameters at various time periods of the study. Investigator VR was also blinded and carried out the radiographic analysis. Prior to the initiation of the study, intra-examiner calibration was achieved for the blinded examiners.
Preparation of 400μg ALN solution: 1% ALN solution was prepared by dissolving 10mg ALN (Osteofos, Cipla Ltd., Roorke, India) in normal saline. A 40μl of this solution contains 400μg of ALN.
Clinical parameters: The soft tissue parameters were recorded to the nearest millimeter with the help of a UNC 15 probe (Hu-Friedy, Chicago, and IL.) and a fabricated customized acrylic occlusal stent with a guiding groove for the probe. The soft tissue parameters of CAL, PD and GR were recorded at baseline, 3 months and 6 months.
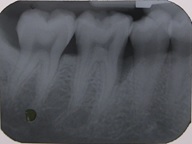
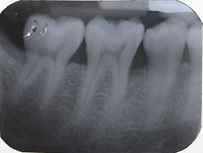
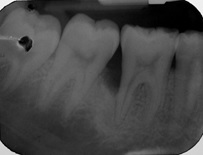
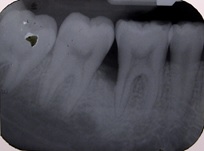
Radiographic parameters: Radiographs at baseline and 6 months were obtained as reproducible as possible using long cone paralleling technique with Rinn XCP device [Table/Fig-1,2,3 and 4].
Baseline radiograph of a test group patient.
Sixth month radiograph of a test group patient.
Baseline radiograph of control group patient.
Sixth month radiograph of control group patient.
The radiographs were digitized and then analyzed using AutoCAD 2006 software (Autodesk, Inc., California,U.S.). The anatomical landmarks of Cemento-Enamel Junction (CEJ), Alveolar Crest (AC), and Base of Defect (BD) were marked on the digitized radiographs based on the criteria set by Schei et al., [32]. The distances from CEJ to BD, CEJ to AC and CEJ to root apex were recorded for the baseline and 6 months radiographs and the radiographic parameters of LBG, %BF and change in Alveolar Crest Height (ACH) were evaluated. LBG was calculated as the difference between the CEJ to BD distance at baseline and CEJ to BD distance at 6 months. The %BF was obtained by dividing LBG by the radiographic defect depth at baseline. The change in the ACH was calculated as the difference between the CEJ to AC at baseline and CEJ to AC at 6 months. For any paired baseline and 6 month radiographs demonstrating any difference in the measurement from CEJ to the root apex of the defect tooth, measurements were corrected accordingly for elongation and foreshortening.
Surgical procedure: Full-mouth scaling and root planing and oral hygiene instructions were given to all the participants prior to the baseline surgical visit. Before the initiation of surgical procedure, the baseline clinical parameters were recorded and the baseline radiograph was taken. Local anesthesia (2% Xylocaine hydrochloride with 1:80000 adrenaline) was administered, a full thickness mucoperiosteal flap was raised, and thorough root planing and debridement were performed [Table/Fig-5].
Intra-osseous defect after debridement.
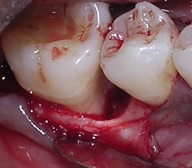
Direct measurements of the intra-bony defect were recorded and final eligibility was confirmed. Based on the size of the defect, an approximate quantity of the β-TCP (Sorbone, Meta Biomed Co., Ltd., Korea) graft material was taken. For the test group patients, 40μl of 1% ALN solution containing 400μg of ALN, was added to the graft material using a micro pipette (Accumax, Fine Care Corporation, Ahmedabad). The ALN soaked β-TCP was allowed to sit for 10 minutes to permit binding of the drug into the β-TCP particles [21]. If wetting of graft was not complete, required quantity of saline was added with care, for complete wetting of the graft so as to get a moldable implantable material [Table/Fig-6].
Implantation of bone graft.
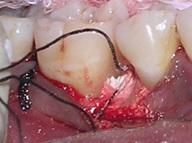
For the control group patients, β-TCP was soaked only in saline and used for implantation. The flaps were sutured using 3-0 braided silk sutures. Appropriate post-operative care and instructions were given and sutures were removed one week later. Throughout the study period patients were monitored for any adverse effects like pain, swelling, inflammation, infection and allergic reactions.
Outcomes: Radiographic hard tissue measurements of LBG, %BF and change in ACH were the primary outcomes. Clinical soft tissue parameters of CAL gain, PD reduction and degree of post-operative gingival recession were the secondary outcomes.
Statistical Analysis
Substituting the values of the primary outcome, linear bone growth, from a similar previous study [27], with a power of 95% and confidence interval of 95%, the sample size was estimated to be five for each group. Considering 10%-20% for attrition, and to detect mean differences of clinical and radiographic parameters between the two groups, a total of 16 in each group were included. All the analysis was done using SPSS version 18. A p-value of < 0.05 was considered statistically significant. Comparison of intra-group values was done using paired t test and repeated measures ANOVA with post-hoc Bonferroni test. Inter-group analysis was done using independent sample t-test.
Results
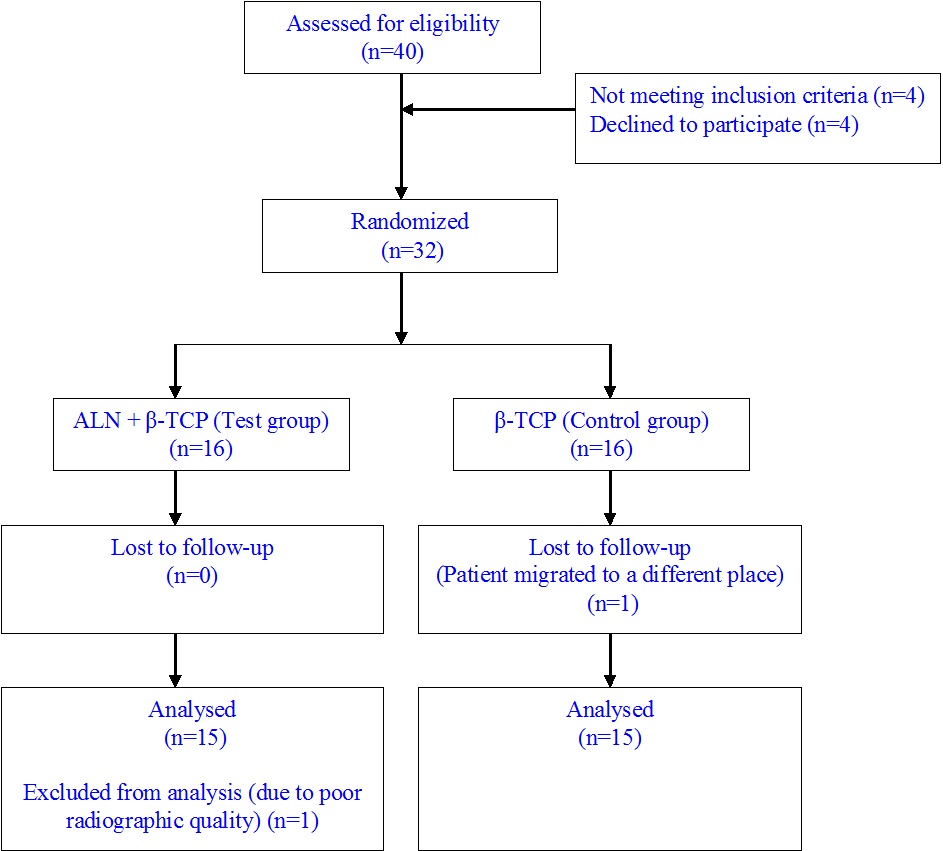
Thirty patients of thirty two enrolled completed the study [Table/Fig-7]. One patient from the control group was lost to follow-up and one patient from the test group was excluded from analysis due to poor radiographic quality. There were no statistically significant differences observed in any of the baseline characteristics such as age, CAL, PD, GR, vertical osseous defect morphology (no. of walls of defect), defect depth, defect width and defect location of the study teeth in both the test and control groups indicating appropriate randomization [Table/Fig-8].
CONSORT patient flow chart.
Baseline characteristics between groups which show no significant difference:
| Baseline Characteristics | Test Group(n=15) | Control Group(n=15) | p-value* |
|---|
| Mean | SD | Mean | SD |
|---|
| Age (years) | 38.00 | 3.34 | 38.93 | 3.39 | 0.454 (NS) |
| CAL (mm) | 9.53 | 1.88 | 9.20 | 1.08 | 0.557(NS) |
| PD (mm) | 9.00 | 1.81 | 8.87 | 1.19 | 0.813 (NS) |
| GR (mm) | 0.53 | 1.55 | 0.33 | 0.49 | 0.640(NS) |
| Osseous defect depth (mm) | 7.54 | 1.80 | 8.00 | 2.28 | 0.610(NS) |
| Osseous defect width (mm) | 3.63 | 0.50 | 3.27 | 0.78 | 0.211 (NS) |
| Defect tooth: Multi-rooted | 7 | 7 | Same values (NS) |
| Single-rooted | 8 | 8 |
| Defect tooth: Multi-rooted | 11 | 12 | Same values (NS) |
| Single-rooted | 4 | 3 |
| Osseous defect morphology |
| 1 walled | 3 | 2 | 0.626(NS) |
| 1.5 walled | 3 | 4 |
| 2 walled | 8 | 7 |
| 3 walled | 1 | 2 |
| Mean ± SD (no.) | 1.77 ± 0.53 | 1.87 ± 0.58 |
*Independent sample t-test
CAL= Clinical Attachment Level; PD = Probing Depth; GR = Gingival Recession; NS = Not Significant.
Intra-group analysis of both the test and control treatment modalities showed significant improvements in the clinical soft tissue parameters and radiographic LBG and %BF [Table/Fig-9,10].
Intra-group analysis for clinical parameters:
| Group | Parameter | Visit | Mean | SD | p-value* | Post-hoc test |
|---|
| Test Group | CAL(mm) | Baseline | 9.53 | 1.88 | <0.001† | B>3>6 |
| 3 months | 6.80 | 1.86 |
| 6 months | 6.13 | 1.88 |
| PD (mm) | Baseline | 9.00 | 1.81 | <0.001† | B>3>6 |
| 3 months | 5.40 | 1.59 |
| 6 months | 4.67 | 1.68 |
| GR (mm) | Baseline | 0.53 | 1.55 | <0.001† | 3,6>B |
| 3 months | 1.53 | 1.41 |
| 6 months | 1.40 | 1.55 |
| Control Group | CAL(mm) | Baseline | 9.20 | 1.08 | <0.001† | B>3,6 |
| 3 months | 7.27 | 1.16 |
| 6 months | 7.00 | 1.07 |
| PD (mm) | Baseline | 8.87 | 1.19 | <0.001† | B>3>6 |
| 3 months | 6.40 | 1.12 |
| 6 months | 5.67 | 1.11 |
| GR (mm) | Baseline | 0.33 | 0.49 | <0.001† | 3,6>B |
| 3 months | 0.87 | 0.74 |
| 6 months | 1.20 | 0.77 |
*Repeated measures ANOVA with post-hoc Bonferroni test
† Statistically significant at p < 0.05
CAL= clinical attachment level; PD = probing depth; GR = gingival recession.
Intra-group analysis for radiographic parameters:
| Group | Parameter | Baseline | 6 months | p-value* |
|---|
| Mean | SD | Mean | SD |
|---|
| Test Group | CEJ-BD (mm) | 8.81 | 1.30 | 5.93 | 1.09 | <0.001† |
| CEJ-AC (mm) | 3.27 | 1.13 | 2.95 | 1.29 | 0.088 |
| Control Group | CEJ-BD (mm) | 8.59 | 1.09 | 6.89 | 0.97 | <0.001† |
| CEJ-AC (mm) | 2.99 | 1.00 | 3.23 | 0.92 | 0.036† |
*Paired t test
† Statistically significant at p < 0.05
CEJ = Cementoenamel Junction; BD = Base Of Defect; AC = Alveolar Crest.
The ALN test group, compared to the control group, showed significantly improved results for mean gain in CAL (p<0.001) and mean reduction in probing depth (p=0.004). The test group showed a mean LBG of 2.88 ± 0.88 mm which was statistically greater than 1.70 ± 0.39 mm obtained by the control group. The ALN test group showed crestal apposition in contrast to the crestal resorption shown by the control group. The %BF was significantly greater for the test group compared to the control group, indicating the effectiveness of ALN in enhancing bone formation [Table/Fig-11].
Inter-group analysis for clinical and radiographic parameters:
| Parameter | Test Group | Control Group | p-value* |
|---|
| Mean | SD | Mean | SD |
|---|
| Change in CAL (mm) baseline to 3 months | 2.73 | 1.10 | 1.93 | 1.10 | 0.056 |
| Change in CAL (mm) baseline to 6 months | 3.40 | 0.74 | 2.20 | 0.86 | <0.001† |
| Change in PD (mm) baseline to 3 months | 3.60 | 1.12 | 2.47 | 1.13 | 0.01† |
| Change in PD (mm) baseline to 6 months | 4.33 | 0.82 | 3.20 | 1.15 | 0.004† |
| Change in GR (mm) baseline to 3 months | 1.00 | 0.85 | 0.53 | 0.52 | 0.079 |
| Change in GR (mm) baseline to 6 months | 0.73 | 0.70 | 0.87 | 0.74 | 0.618 |
| LBG: Change in CEJ-BD (mm) baseline to 6 months | 2.88 | 0.88 | 1.70 | 0.39 | <0.001† |
| Change in CEJ-AC (mm) baseline to 6 months | 0.32 | 0.68 | -0.24 | 0.40 | 0.011† |
| %BF (%) baseline to 6 months | 51.98 | 15.84 | 30.35 | 6.88 | <0.001† |
*Independent sample t test
† Statistically significant at p < 0.05
CAL= clinical attachment level; PD = probing depth; GR = gingival recession; CEJ = cementoenamel junction; BD = base of defect; AC = alveolar crest.
Discussion
The hypothesis tested in this study was that the combining of a host modulating agent, ALN, with a widely used osteoconductive grafting carrier material, β-TCP, would lead to favorable clinical and radiological outcomes. Osteofos used in the present study is easily available by prescription and very economical (Rs.5.48 per 10mg tablet). The addition of 400μg of it to β-TCP bone substitute has enhanced the bone formation potential. The ALN test group was more effective for all the parameters evaluated except for post-operative gingival recession, which was same in both the test and control groups [Table/Fig-11]. A parallel-arm design with each patient contributing one defect was selected, instead of a split mouth design, as Yaffe et al., in a rat model demonstrated that 10% of the topically applied 200μg radiolabelled ALN was absorbed by the alveolar bone at the experimental site and 20% to 30% of this absorbed ALN reached the alveolar bone on the contralateral side by systemic disposition [33]. Since ALN was delivered with β-TCP, an active-control, i.e., β-TCP, was employed in the study to prove the efficacy of ALN, instead of open flap debridement.
Binderman et al., showed that both 200μg and 400μg were effective in reducing bone loss following flap surgery in rats, but 400μg ALN was less effective than 200μg. They stated that 400μg possibly increased local acidity and thus interfered with anti-resorptive activity. However, the surface area of bone exposed to the drug in the rat model would be very minute compared to the human periodontal defects. Hence, in the present study 400μg dosage was utilized. Moreover, the drug was entrapped in β-TCP and was released over a period of time.
The present study showed a significant gain in CAL, reduction in PD as seen in previous studies which used ALN gel as Local Drug Delivery (LDD) [28–30]. There was a significant increase in bone formation in the test group of the present study, which is in accordance with Srisubut et al., who demonstrated histologically the bone formation potential of ALN in rats using bioactive glass soaked in 2% ALN [15]. It was shown in their study that ALN did not cause cell death of osteoclasts, but only inhibited their function. Gupta et al., on using synthetic hydroxyapatite alloplast in conjunction with ALN obtained a higher mean LBG of 3.6 ± 0.29 compared to the 2.88 ± 0.88 of the present study [27]. The LBG and %BF obtained by the ALN test group in the present study was higher than that obtained by Sharma et al., in the treatment of chronic periodontitis using 1% ALN gel as LDD [28]. This increase in the radiographic hard tissue parameters in the present study could be due to the additional access flap surgery and the use of an osteoconductive bone graft material as a delivery vehicle for ALN.
There was no alveolar crestal resorption in the ALN test group which is in accordance to the findings of Binderman et al., Kaynak et al., and Fleish et al., that ALN has selectively high affinity to adsorb to bone mineral at the sites of active bone remodeling, especially the surgical wounds [12,34,35].
The binding of bisphosphonates to the calcium phosphates is due to the chemical interaction between them by the phosphate groups and also due to surface adsorbtion by electrostatic interactions [19]. The strong bonding of the first layer of bisphosphonates and β-TCP results from a chemical exchange of the phosphate group [36]. Subsequent layers of the drug bind to the β-TCP particles surface by weak electrostatic forces [24]. An initial burst release is due to diffusion of the drug adsorbed by weak electrostatic forces and a later slow release of the chemically bound drug occurs by the matrix degradation of the β-TCP.
Verzola et al., showed that ALN therapy resulted in enhanced osseointegration of implants in a rat model [37]. However, contrary to their finding, Guimarães et al., in a rabbit model showed that ALN therapy did not favour osseointegration of implants [38]. Avascular osteonecrosis of the jaw has been reported in cancer and osteoporosis patients who were on long-term, systemic administration of bisphosphonates [39]. The present study used a single, local, low concentration of ALN and no adverse effects were found in any of the patients throughout the study period. Both the anti-resorptive and bone regenerative potential of ALN can be tapped and used as a treatment modality for periodontal defects.
Limitation
The present, short, six-month study had a small sample size, hence, further long term studies with larger sample size have to be performed focusing on the dosage of ALN and the long term prognosis of ALN on bone remodeling.
Conclusion
The present study showed that the predictability of bone formation increased by adding a host modulating agent like ALN to bone graft materials. Histologic studies are required to confirm the bone formation potential and effective dosage of ALN in human intra-osseous defects. Further research to produce an efficient drug delivery vehicle for controlled sustained release of the drug is required.
*Independent sample t-test
CAL= Clinical Attachment Level; PD = Probing Depth; GR = Gingival Recession; NS = Not Significant.
*Repeated measures ANOVA with post-hoc Bonferroni test
† Statistically significant at p < 0.05
CAL= clinical attachment level; PD = probing depth; GR = gingival recession.
*Paired t test
† Statistically significant at p < 0.05
CEJ = Cementoenamel Junction; BD = Base Of Defect; AC = Alveolar Crest.
*Independent sample t test
† Statistically significant at p < 0.05
CAL= clinical attachment level; PD = probing depth; GR = gingival recession; CEJ = cementoenamel junction; BD = base of defect; AC = alveolar crest.