Disinfectants used in hospitals and clinical laboratories should be tested before being introduced into routine use [1]. Disinfection process validation is the establishment of documented evidence that a disinfection process will consistently remove or inactivate known or possible pathogens from inanimate objects [2]. Most commercially available disinfectants claim good efficacy and certifications regarding disinfection process validation at various specified concentrations [3]. In our hospital, we use steam sterilization and ethylene oxide for critical items, 2.4% Glutaral-dehyde for semicritical high level disinfection and 70% ethyl alcohol for non-critical items. Distributors of other commercially available disinfectants questioned our policy claiming greater efficacy and lack of toxicity of their products. They provide certifications assessing the quality of their products; validity and quality levels of which are not known to end users [3]. Some of these certifications may be based on inadequate testing [4,5]. Further, aldehyde based disinfection; like that used in our hospital has been linked to occupational asthma, dermatitis, irritation to eyes, and colitis [1,6]. Indeed, some health care professionals advocate aldehyde free disinfectants for semi critical high level disinfection [7]. On the other hand, inadequate disinfection procedures may lead to high morbidity, economic burden on the patient, and ultimately the risk of mortality [1,3,8]. We therefore conducted an experiment testing the efficacy of various forwarded commercial disinfection products before incorporating any of them in our hospital protocol.
Materials and Methods
This laboratory based experimental study was conducted at St. Stephen Hospital, Delhi during July-September 2013. The disinfectants tested were: D1-Sanidex® (0.55% Orthopthalaldehyde), D2-Sanocid® (1% Glutaraldehyde + 1.2% QID), D3-Cidex® (2.4% Glutaraldehyde), D4-2% SekuSept Aktiv® (1% Sodium perborate Monohydrate, 0.5% Tetraacetylenediamine), D5-5% BIB Forte® (dodecyldipropylenetriamine, trialkylethoxyammoniumproprionate, tenosides & auxillaries), D6-5% Alprojet W® (Amidosulfonic acid, NaHPO4, tenside auxillaries), D7-2% Desnet® (Didocyldimethyl Ammonium chloride, Sodium carbonate), D8- 5% Sanihygiene® (polyhexamethylene biguanide + QAC), D9-Incidin® (0.5% benzalkonium chloride, Didocyldimethyl ammonium chloride), D10-1.56% D125® (Alkyl (60% C14, 30% C16, 5% C12, 5% C18) dimethyl benzyl ammonium chloride 2.37%, Alkyl (68% C12, 32% C14) dimethyl ethylbenzyl ammonium chloride 2.37%), D11-Lonzagard® (2% Bis-3-aminoguanyl dodecyl ammonium chloride), D12-Glutishield® (2.0% Glutaraldehyde). Recommended strength disinfectant solutions were freshly prepared. Tests were conducted at 25±1o C and pH of media and normal saline was 7.0±0.2; disinfectant solutions (25ml) were aliquoted in sterile universal containers and placed in 25o C waterbath for temperature equilibration [3]. Test microorganisms used were: Staphylococcus aureus ATCC®25923, Escherichia coli ATCC®25922, Salmonela Typhi clinical isolate (blood culture isolate B-803/2013 from patient), Pseudomonas aeruginosa clinical isolate (sputum isolate P-315/2013 from patient), Bacillus cereus (External Quality Assurance isolate from Christian Medical College, Vellore) and Mycobacterium fortuitum clinical isolate {wound exudate, identification confirmed by Dr Mandira Varma Basil by PCR restriction analysis (method of Wong et al., 2001) [9] and phenotypic method}. Two inoculums were prepared in sterile normal saline {High Inoculum (HI) (McFarland No.3~9*108 cfu/ml), Low inoculums (LI) (McFarland No0.5~1.5*108 cfu/ml)} for each bacterium except Mycobacterium fortuitum for which only LI was prepared. Turbidity was measured in a turbidometer. A 250 μl of each inoculum was added to the respective labelled disinfectant containers and well mixed, giving final concentrations of 9 X 106 and 1.5 X 106 cfu/ml. 500 μl of the test solutions were neutralized in 4.5 ml sterile Nutrient broth at 5min, 10min, 20min, 30min and 2 hours since addition of bacteria [3]. After 10 minutes of neutralization, 10μl was sub-cultured on Nutrient Agar (Sheep Blood agar for Mycobacterium fortuitum) in duplicates. One set was incubated at 370C and the other at 250C for 10 days and inspected daily for growth. Colonies were identified by morphology, Gram stain, Ziehl-Neelsen stain and routine biochemical tests. Colonies from each subculture were enumerated and logarithmic reductions were calculated. All tests were repeated twice to verify the reproducibility of the findings, and mean values were taken as final results. Inhibition of Bacillus cereus was taken as evidence for high-level disinfection, inhibition of Mycobacterium fortuitum for intermediate-level disinfection and that of the other four for low-level disinfection. According to chemical composition, disinfectants were grouped and compared. D1, D3 and D12 were aldehydes, D4 and D6 oxidizing agents; D5, D7, D9, D10, and D11 Quarternary ammonium compounds (QACs); D2 and D8 combination of two chemical types. For statistical analysis, an average of four observations (two each at 37° and 25°C) was taken for calculating log reduction achieved by the disinfectant for each organism. Log reduction at 2 hours after adding disinfectant was calculated by using the following formula [3]:
Log10 Reduction (R) at end point (2 hours) = Log10 Prevalue cfu/ml - Log10 Postvalue cfu/ml.
Results
All twelve disinfectants tested were strongly active against Salmonella Typhi (both high and low Inoculum) reducing counts to zero within 5 minutes [Table/Fig-1] Log10 (cfu/ml) reduction in bacterial growth at various exposure points with different categories of disinfectants are depicted graphically ([Table/Fig-2]: aldehydes and oxidizing agents and [Table/Fig-3]: QACs and combination disinfectants). Against S. aureus and E. coli, all disinfectants except D6 reduced counts to zero in 5 minutes. D6 (Alprojet W®) took 30 and 10 minutes to reduce HI {R (Log10 reduction) =5.95} and LI (R=5.18) S. aureus counts to zero respectively. Further D6 worked poorly against Escherichia coli taking 20 minutes to reduce counts to zero in both high (R=5.95) and low inoculum (R=5.18) [Table/Fig-1,2f]. Killing of low inoculum Pseudomonas aeruginosa was achieved by all disinfectants at 5 minutes while against HI-Pseudomonas, D7 (Desnet®) and D10 (D-125®) took 20 minutes (R=5.95) to achieve zero counts [Table/Fig-1,3c]. From these findings it can be ascertained that D6, D7, and D10 took inordinately long time as low level disinfectants in various situations.
Average (370C & 250C, tested in repeats) number of colony forming units / ml of each species of bacteria at various point of time of treatment with disinfectants
| Disinfectant | Bacteria | Average of Colony counts at 370C & 250 C |
|---|
| High Inoculum | Low Inoculum |
|---|
| 5 min | 10 min | 20 min | 30 min | 2 hours | 5 min | 10 min | 20 min | 30 min | 2 hours |
|---|
| D1 | Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D6 | 16 x103 | 10 x103 | 9.5 x103 | 0 | 0 | 6 x103 | 0 | 0 | 0 | 0 |
| D7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D1 | Escherichia coli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D6 | 16x103 | 7.5x103 | 0 | 0 | 0 | 6.25 x103 | 2.75 x103 | 0 | 0 | 0 |
| D7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D1 | Salmonella Typhi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D1 | Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D6 | 0.05x103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D7 | 17.5x103 | 15 x103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D10 | 10x103 | 1.35 x103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D1 | Bacillus cereus | 3 x103 | 1.25 x103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D2 | 2.75 x103 | 2.5 x103 | 0.1 x103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D4 | 17.5x103 | 10x103 | 1.5x103 | 0.65x103 | 0 | 0.65x103 | 0.1x103 | 0 | 0 | 0 |
| D5 | 4.25x103 | 3.6x103 | 5.6x103 | 1.25x103 | 0.6x103 | 2.35x103 | 1.9x103 | 1.65x103 | 1.5x103 | 1x103 |
| D6 | 20x103 | 17.5x103 | 3.5x103 | 3.25x103 | 2.5x103 | 7x103 | 3.1x103 | 2.65x103 | 2.25x103 | 1.15x103 |
| D7 | 20x103 | 17.5x103 | 1.45x103 | 1.7x103 | 0.95x103 | 2.05x103 | 2.25x103 | 0.55x103 | 0.5x103 | 0.3x103 |
| D8 | 15x103 | 12x103 | 7.75x103 | 6.5x103 | 2.1x103 | 2.05x103 | 2.25x103 | 0.55x103 | 0.5x103 | 0.3x103 |
| D9 | 15x103 | 12x103 | 7.5x103 | 7x103 | 2.35x103 | 3.05x103 | 1.6x103 | 1.1x103 | 1.05x103 | 2x103 |
| D10 | 9x103 | 10x103 | 2.35x103 | 4.25x103 | 0.95x103 | 2.25x103 | 2.2x103 | 1.75x103 | 0.95x103 | 0.05x103 |
| D11 | 6.5x103 | 3.5x103 | 3.35x103 | 3.25x103 | 0.7x103 | 0.5x103 | 0.2x103 | 0.2x103 | 0.1x103 | 0.85x103 |
| D12 | 1.7x103 | 1.5x103 | 0.7x103 | 0.55x103 | 0 | 0.15x103 | 0 | 0 | 0 | 0 |
| D1 | Mycobacterium fortuitum | NT | NT | NT | NT | NT | 0 | 0 | 0 | 0 | 0 |
| D2 | NT | NT | NT | NT | NT | 0 | 0 | 0 | 0 | 0 |
| D3 | NT | NT | NT | NT | NT | 0 | 0 | 0 | 0 | 0 |
| D4 | NT | NT | NT | NT | NT | 14.5x103 | 5.5x103 | 2.6x103 | 0.6x103 | 0.05x103 |
| D5 | NT | NT | NT | NT | NT | 0.85x103 | 0 | 0 | 0 | 0 |
| D6 | NT | NT | NT | NT | NT | 22x103 | 23.5x103 | 25x103 | 19.25x103 | 16.5x103 |
| D7 | NT | NT | NT | NT | NT | 17.5x103 | 15x103 | 10.5x103 | 9.5x103 | 0.5x103 |
| D8 | NT | NT | NT | NT | NT | 18.75x103 | 35x103 | 20x103 | 13.5x103 | 0.95x103 |
| D9 | NT | NT | NT | NT | NT | 35x103 | 30x103 | 14x103 | 15.5x103 | 0.85x103 |
| D10 | NT | NT | NT | NT | NT | 35x103 | 25x103 | 20x103 | 15x103 | 1.1x103 |
| D11 | NT | NT | NT | NT | NT | 0 | 0 | 0 | 0 | 0 |
| D12 | NT | NT | NT | NT | NT | 0 | 0 | 0 | 0 | 0 |
Footnotes- D1-Sanidex, D2-Sanocid, D3-Cidex, D4-2% Seku Sept Aktive, D5-5% BIB Forte, D6-5% Alprojet W, D7-2% Desnet, D8- 5% Sanihygiene, D9-Incidin, D10-1.56% D125, D11-Lonzagard, D12-Glutishield, NT – Not tested
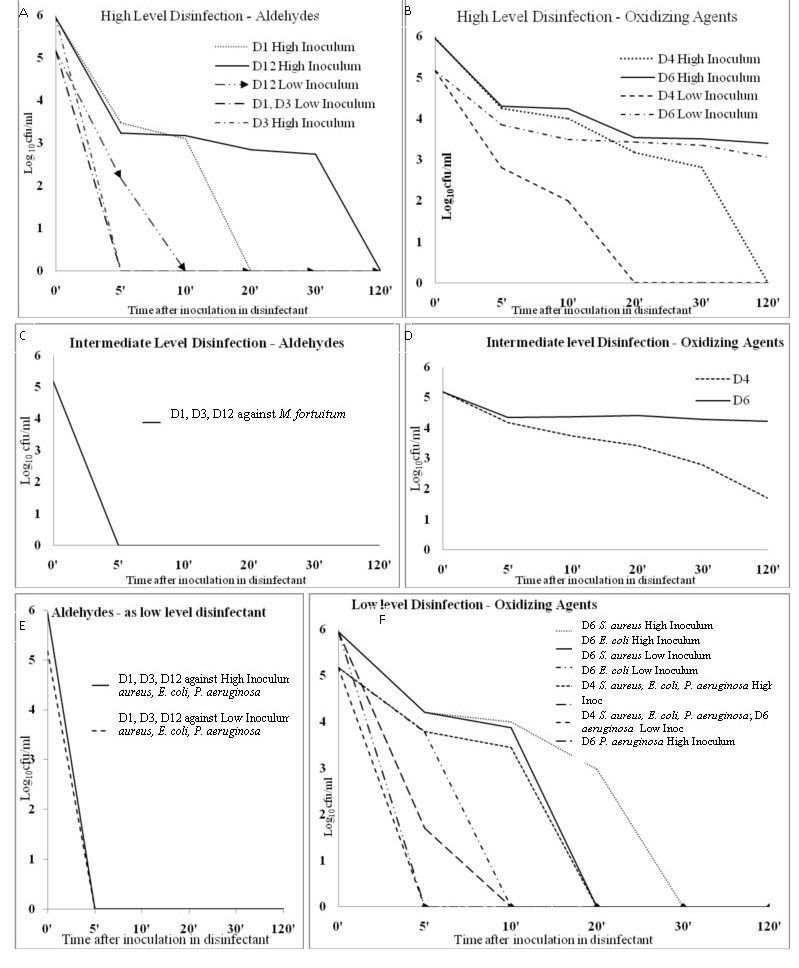
Log10 (colony forming units/ml) reduction in bacterial growth (both high and low inoculums) at various exposure points with aldehyde (D1, D3, D12) and oxidizing agent (D4, D6) groups of disinfectants.
A: Aldehydes: D1 (Sanidex®), D3 (Cidex®), and D12(Glutishield®) as High-level disinfectant.
B:Oxidizing Agents: D4 (Seku Sept Aktive®) and D6 (Alprojet W®) as High-level disinfectant.
C: Aldehydes: D1, D3, and D12 as Intermediate-level disinfectant.
D: Oxidizing Agents: D4 and D6 as Intermediate-level disinfectant.
E: Aldehydes as low level disinfection (all four bacteria E. coli, S. aureus, S. Typhi and P. aeruginosa showed zero growth at 5 minutes and beyond hence not shown separately).
F: Oxidizing Agents: D4 and D6 as Low-level disinfectant.
Inoc = Inoculum
Log10 (colony forming units/ml) reduction in bacterial growth (both high and low inoculums) at various exposure points with Quarternary Ammonium compund (D5, D7, D9, D10, D11) and combination (D2, D8) groups of disinfectants.
A: Quarternary Ammonium Compunds: D5 (BIB-Forte®), D7(Desnet®), D9 (Incidin®), D10 (D125®), D11 (Lonzagard®) as High-level disinfectant.
B: Quarternary Ammonium Compunds: D5, D7, D9, D10, D11 as Intermediate-level disinfectant.
C: Quarternary Ammonium Compunds: D5, D7, D9, D10, D11 as Low-Level disinfectant (against Pseudomonas aeruginosa only; against Staphylococcus aureus, Salmonella Typhi and Escherichia coli zero counts were recorded for all disinfectants at 5 minutes and beyond).
D: Combination disinfectants: D2 (Sanocid®) and D8 (Sanihygiene®) as High-level disinfectant and as Intermediate-Level disinfectant. (both D2 and D8 as low level-disinfectant, reduced S. aureus, E. coli, S. Typhi and P. aeruginosa to zero counts at 5 minutes and beyond, hence not shown in figure). Inoc = Inoculum
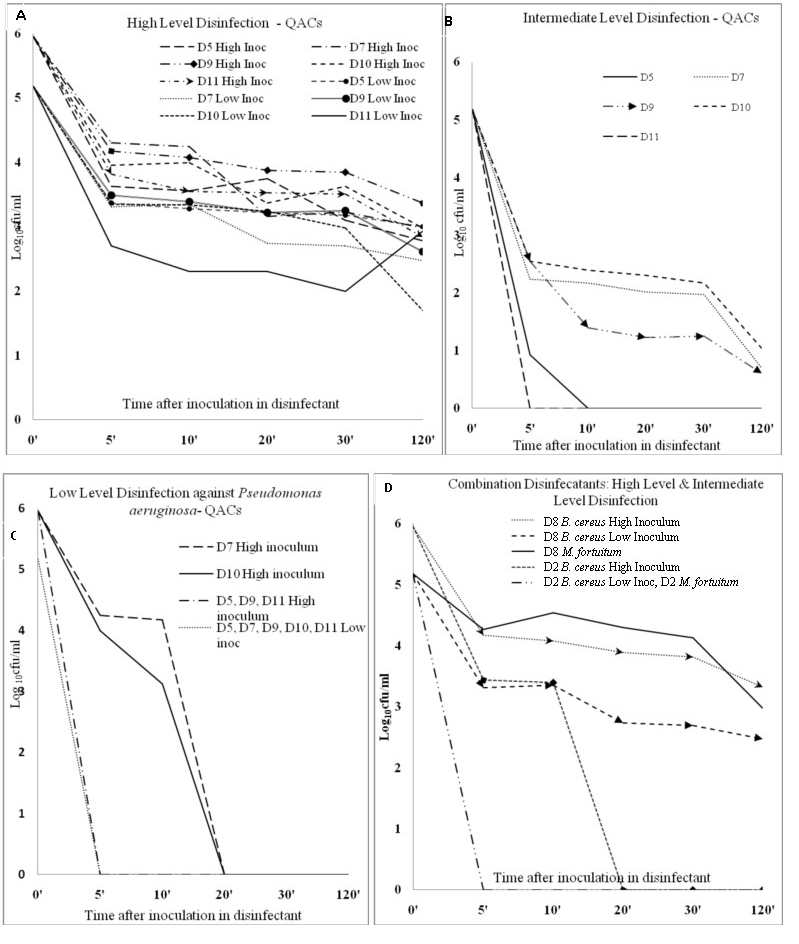
Against Mycobacterium fortuitum D1 [Table/Fig-2c], D2 [Table/Fig-2d], D3 [Table/Fig-2c], D11 [Table/Fig-3b], and D12 [Table/Fig-2c] were able to inhibit at 5 min, while D4 (R=3.48), D6 R=(0.96) [Table/Fig-2d], D7 (R=4.48) [Table/Fig-3b], D8 (Sanihygiene®) (R=2.20) [Table/Fig-3d], D9 (R=4.56), and D10 (R=4.13) [Table/Fig-3b] were unable even at 2 hours. D5 [Table/Fig-3b] took 10 minutes to reduce Mycobacterium fortuitum counts to zero (R=5.18).
For sporicidal activity testing against Bacillus cereus only D3 (Cidex®) reduced counts to zero in 5 minutes (HI and LI) [Table/Fig-2a]. Surprisingly D5 (BIB Forte®) (R=2.18) [Table/Fig-3a], D6 (R=2.12) [Table/Fig-2b], D7 (R=2.70) [Table/Fig-3a], D8 (R=2.70) [Table/Fig-3d], D9 (Incidin®) (R=2.56), D10 (R=3.48), and D11 (Lonzagard®) (R=2.25) [Table/Fig-3a] were unable to inhibit the bacterium after 2 hours even at low inoculum. Bacillus cereus counts showed a paradoxical increase at various time points when treated with three of the QACs. (High inoculum with D5 at 20 minutes, High inoculum with D10 at 30 minutes and Low inoculum with D11 at 2 hours) [Table/Fig-1]. D1 [Table/Fig-2a] and D2 [Table/Fig-3d] achieved zero counts for LI-Bacillus cereus at 5 min but took 20 and 30 minutes respectively for High inoculum-Bacillus cereus. D4 [Table/Fig-2b] and D12 [Table/Fig-2a] reduced counts to zero after 20 and 10 minutes respectively for LI-Bacillus cereus and two hours for high inoculum of the same bacterium. These findings attest D3 (Cidex®, 2.4% gluteraldehyde) as being the best among the twelve disinfectants, whether it be for high level (sporicidal), intermediate level (anti-mycobacterial) or low level purposes. In contrast, D-125® and Alprojet W® fared the worst.
Discussion
Disinfectant testing can be broadly categorized into four groups; carrier tests, suspension tests, capacity tests and practical tests [10]. Of these we used a standardised quantitative suspension test, the disinfectant kill time test to evaluate twelve commercially available disinfectants. This type of quantitative suspension test is simple to perform and is the most basic of disinfectant tests required by French, German and European authorities on disinfection [10].
Disinfection processes constitute an essential part of hospital infec-tion control services that eliminates most pathogenic microorganisms, except bacterial spores, on inanimate objects [1]. In hospitals, Spaulding criteria are used to categorize instruments and surfaces and implement disinfection accordingly [11]. Many critical (laprascopes and arthroscopes) and semicritical (endoscopes, respiratory therapy and anaesthesia equipment, laryngoscope blades, oesophageal manometry probes, cystoscopes, anorectal manometry catheters) items used in our hospital are treated by high level disinfectants. The intricate device design makes cleaning such devices difficult and heat sensitive parts makes sterilization by heat nonviable [11]. Further many semicritical instruments are used while performing invasive procedures eg, endoscopic biopsy [11]. Thus disinfection processes undertaken in the hospital are critical steps in preventing Hospital Acquired Infections (HAIs). Increasingly, a number of new disinfectants are available claiming increased efficacy and reduced toxicity over older ones. However, before introduction in hospitals their efficacy must be validated by a suitable combination of disinfectant tests [10]. Our study shows that despite claims to the contrary, many new commercial disinfectants failed to provide minimal satisfactory disinfection. Some were incapable of providing even low level disinfection at appropriate interval of time. The FDA calls for a high-level disinfectant to achieve a 6-log10 kill of an appropriate Mycobacterium species in short contact time [11]. However, some disinfectants despite killing Mycobacterium fortuitum were unable to get rid of Bacillus cereus.
Aldehydes were efficacious high-level disinfectants during our experiments; Cidex® and Sanidex® having sporicidal effect within reasonable time (5 minutes and 20 minutes at high inoculum). Expectedly, QACs as is known from literature [1–3] fared poorly in this regard. Among the aldehydes, Cidex® (2.4% Glutaraldehyde) fared best, followed by Sanidex® (0.55% ortho-pthaladehyde) and then Glutishield® (2% Glutaraldehyde). This emphasizes that concentration of Glutaraldehyde used is important for semi-critical disinfection, a level of ≥2.4% being suitable [8]. Below 2.4% concentration, Glutaraldehyde should not be used alone but be combined with a phenol or similar compound to achieve high level disinfection [11]. We found ortho-pthalaldehyde (OPA) took more than 10 minutes to achieve 4 log10 sporicidal activities; beyond the time specified for disinfection by some manufacturer labels in European, Asian and Latin American countries (5 minutes) [11]. Though 5 minutes was enough for mycobactericidal action which is the benchmark for intermediate level disinfection, longer time required for sporicidal activity should also be considered when disinfecting endoscopes and other semi-critical items. Anaerobic spore bearing bacteria are residents of the intestine. We suggest that it would be more prudent to follow US guidelines by CDC to allow 12 minutes contact time for OPA containing disinfectants [11]. OAs in our study, D4 and D6, were incapable of any sporicidal action. We therefore suggest that SekuSept Aktiv® and Alprojet W® should therefore not be used for high level disinfection.
As a marker of intermediate level disinfection, activity against Mycobacterium fortuitum was very high for the aldehyde disinfectants; all three effectively eliminating M. fortuitum in 5 minutes. OAs took inordinately long to have any effect on mycobacteria, negating their usefulness for any semicritical disinfection procedure. In fact, D6 could not even achieve a 2-log10 reduction in Mycobacterium fortuitum counts after 2 hours. As per our findings, SekuSept Aktiv® and Alprojet W® were incapable of intermediate level disinfection and should not be used for any semi-critical disinfection. QACs, apart from being poorly sporicidal [1–3,11], are also poor mycobactericidal [12], as also documented by us (< 3-log10 reduction even after 120 minutes).
Among the pathogenic vegetative bacteria tested, Salmonella Typhi and S. aureus were eliminated in our experiments by all the QAC and aldehyde disinfectants within 5 minutes. In fact, S. Typhi seems to be the most susceptible microbe in our disinfection experiments. We suggest that S. Typhi should not be used as a sole marker disinfection process validation. QACs are known to be poorly effective against Pseudomonas aeruginosa and also other gram negative microbes [1]. Two (Desnet® and D125®) of five QAC brands took 20 minutes to inhibit this organism. Such a long contact period is neither practical nor recommended for disinfection of environmental surfaces [8]. During low level disinfection, a disinfectant is usually applied and allowed to act for ~1-2 minute before normal work resumes. Reports of outbreaks of HAIs from contaminated QAC solutions used on cystoscopes or cardiac catheters [10,12] highlight their lack of effectiveness. QACs have been shown responsible for disinfection failures on multiple occasions leading to HAIs [13,14]. In a previous study based on surface disinfection, D7 had performed well against Pseudomonas aeruginosa [3]. A different principle (surface disinfection in contrast to our time-kill assay), methodology, and resistance of the specific clinical isolate may have contributed to discrepant results in our experiment. Newer generation QACs in D9, D10, and D11 quickly inhibited Pseudomonas aeruginosa and were effective against the other two Gram negatives also. These newer generation QACs are more efficacious, odourless, colourless, noncorrosive and highly stable compounds over a wide range of pH (3–10.5) and temperature [3]. From our data, we suggest that D125® not to be used as low level disinfectant; the role of Desnet® requires further validation studies in the future. Out of the oxidizing disinfectants, D4 was a good low level disinfectant but D6 was disappointing, requiring half an hour or more for doing the same. Alprojet W® should therefore not be used for low level disinfection as it requires prolonged time to kill S. aureus, E. coli and P. aeruginosa.
Our experience regarding potent activity of QAC disinfectants against S. aureus and Salmonella is shared by other workers [15,16]. Seven of eight QAC disinfectants tested by Rogers JD et al., were cidal to S. aureus [15]. Stringfellow K et al., attested to the ability of QACs to kill both S. aureus and Salmonella Typhimurium in the absence of organic matter [17]. Our experimental finding of QACs being ineffective against certain Gram negative bacteria, in our case being P. aeruginosa is documented by others [3,17]. We found commercial oxidizing disinfectants especially Alprojet W® disappointing. Ineffectiveness of other commercial oxidizing disinfectants against vegetative bacteria has also been demonstrated previously [3,15]. Rogers JD et al., found two chlorine-based commercial disinfectants less effective against S. aureus under test conditions [15] while Singh M et al., found Clea-N-Sept® less effective against Klebsiella pneumonia and Salmonella Typhi [3]. In contrast to these, 7.5% H2O2 and 0.23% peracetic acid are among superior disinfectants though belonging to the same group of oxidizing disinfectants [11]. In our study both SekuSept Aktiv® and Alprojet W® were poorly mycobactericidal; similarly poor mycobactericidal activity (after 1 hour contact) of another oxidizing disinfectant, 2-butanone peroxide has been recently demonstrated [18]. Because of this difference between commercial oxidizing disinfectants and their claims, we suggest that any branded oxidizing disinfectant requires pre-introduction validation by the microbiology laboratory. Our finding of rapid efficacy of gluteraldehyde based disinfectants against S. aureus, E. coli and P. aeruginosa is corroborated by others [3,19]. Our experiments and those of March JK et al., [20] have shown that gluteraldehyde takes shorter time to sporicidal activity than OPA based disinfectants. This is despite the latter being an equal [21] if not more effective mycobactericidal agent [1,10]. We demonstrate four out of five QAC brands to be ineffective against mycobacteria while the remaining brand (BIB Forte®) was effective after 10 minutes. Not surprisingly, Bello T et al., found two QAC disinfectants ineffective against M. tuberculosis, M. abscessus and M. chelonae even after 1-hour exposure [21].
The paradoxical increase in Bacillus cereus counts at various time points when treated with D5, D10 and D11 (all QACs) is interesting. This phenomenon could be due to hormetic effect of low levels of QACs on the sporulating bacteria. Bacterial growth instead of being inhibited is augmented in these circumstances by low levels of antimicrobial agents [22,23]. Though known to occur with Pseudomonas aeruginosa and lesser so with Staphylococcus aureus [22], it has not been studied with Bacillus cereus. Similar time dependant hormetic effects are seen with ionic liquids, 1-alkyl-3-methylimidazolium chloride on Vibrio qinghaiensis [23]. Interestingly, during our experiment, Pseudomonas aeruginosa and Staphylococcus aureus were inhibited in a time dependant manner by the recommended levels of QACs unlike Bacillus cereus.
The combination of the biguanide and QAC had a very poor high-level or intermediate-level disinfectant activity. The commercial combination 1% Glutaraldehyde and QAC was poorly sporicidal (at 20 minutes) probably because of subinhibitory concentration of each of the active contents. None of these combinations have been recommended by the CDC&P in their manual [11] on disinfection but are commercially available.
Limitations
We could not evaluate disinfectants for anti-anaerobic, anti-virucidal, and anti-protozoal activities. Though mycobactericidal activity was tested, yet specific anti-tuberculicidal activity was not tested.
Conclusion
Cidex® (2.4% Glutaraldehyde) is a good high-level disinfectant while newer QACs (Incidin®, D125®, and Lonzagard®) were capable low-level disinfectants available at our hospital among the twelve commercially available ones. Use of older QACs and unregulated combination disinfectants is strongly recommended against.
Declaration of Interest
No funds were taken from any source for conducting this study. No commercial relationship or conflict of interest exists for this study or publication. The manuscript in total or in part has not been published or presented elsewhere.
Footnotes- D1-Sanidex, D2-Sanocid, D3-Cidex, D4-2% Seku Sept Aktive, D5-5% BIB Forte, D6-5% Alprojet W, D7-2% Desnet, D8- 5% Sanihygiene, D9-Incidin, D10-1.56% D125, D11-Lonzagard, D12-Glutishield, NT – Not tested