Dental caries is the most common preventable oral disease known to mankind but unfortunately people are susceptible to this disease throughout their lifetime. It is the primary cause of oral pain and tooth loss. Dental caries can be arrested and is reversible in its early stages, but is often not self-limiting. Without preventive measures, proper care and clinical intervention caries can progress until the tooth is completely destroyed. The terms dental caries or caries can be used to identify both the caries process and the carious lesion (cavitated or non-cavitated) that is formed as a result of that process. Based on chronology dental caries is classified into early childhood caries, teenage caries and adult caries [1].
Early Childhood Caries (ECC) is a destructive, debilitating and the most common chronic childhood disease that affect the young children. Not life-threatening but it affects normal health and well-being of the child. Though dental caries prevalence has reduced worldwide, ECC prevalence is still high and is currently a WHO concern [2,3]. Severe ECCC as the name indicates is a severe, aggressive and debilitating form of this disease with ramifications.
Materials and Methods
This cross-sectional study was conducted among 40 healthy children between 12-71 months of age by randomly selecting healthy children reporting to the Department of Pedodontics and Preventive Dentistry, A.J. Institute of Dental Sciences, Mangaluru, Karnataka, India. Children on antifungal or steroid therapy, with positive medical history and those for whom informed parental consent was not obtained were not included in the study. This study was conducted after the Institutional Ethical Committee reviewed and approved the study protocol.
The children included in our study were clinically examined and were divided into two groups consisting of 20 children each based on their caries experience, in accordance to the AAPD definition for SECC [11], i.e., the SECC group was the study group and the caries free group was the control group. Both groups were further divided into Subgroups A and B of 10 children each based on age, i.e., Subgroup A consisted of children between 12-36 months and Subgroup B consisted of children between 37-71 months of age.
To maintain consistency in the sample collected, we used the unstimulated, whole saliva (that pools in the floor of the mouth). It was collected by the passive drool technique and we used swabbing technique which often allows for studies with small children or other individuals that have difficulty with the passive drool technique because of their age and incompetency to cooperate. A sample of 2 ml unstimulated whole saliva was collected between 10:00am to 11:00am by both making the child to drool and also by swabbing technique. Children were made to sit in a well-lit quiet room and made to drool into sterile containers for 10 minutes. Drooled saliva sample was additionally supplemented with swabbed saliva and supragingival plaque sample, which was collected carefully by swabbing the buccal surfaces of the teeth, carious lesions and around the mucosal surface with sterile, absorbent cotton swab until saturated. Swab was then placed into a 5cc syringe and was squeezed using plunger of the syringe. Each sample was labeled and stored at -70oC and transported to the microbiology laboratory for microbiological evaluation.
The saliva samples were centrifuged at 8000rpm for one minute at room temperature. The supernatant was discarded, and 100ul normal saliva was added to the precipitate. The precipitate was then placed in Vortex mixer for 30 seconds to obtain homogenous suspensions. A 10ul of the suspension was inoculated in the chromagar medium by painting with cotton swab to spread the inoculums (Hi Media Laboratories Pvt., Ltd., Code No- M1297A, Mumbai, India) and incubated at 37oC under aerobic conditions for 48 hrs. Isolates were identified according to the different colors of colonies on the agar medium. Candida albicans appeared as green colored smooth colonies, C. glabrata appeared as purple colored smooth colonies, C. tropicalis appeared as dark blue grey colored smooth colonies, C. krusei appeared as pale pink colored smooth colonies. Candida was identified by employing API- 20C AUX and germ tube formation test.
Statistical Analysis
The data was statistically analyzed by using descriptive statistics, ‘Chi-Square’ test and ‘Mann- Whitney U’ test in SPSS software 17.0. The results were considered statistically significant at 0.05 probability levels.
Results
The mean age of the study participants was 3.189 years and in the Subgroups A and B it was 2.08 years and 4.29 years respectively. In the SECC group, the mean ± SD of dfs index was 18.6 ± 11.67, with a minimum of 5 and the maximum of 46. All the study participants included in the SECC group had active caries, for the defs index, score here was indicative of only the d component; and there were no filled or missing teeth due to caries.
[Table/Fig-1a,b] shows the Candida albicans count in both the SECC group and caries free group. Median Candida albicans of the SECC group was numerically greater than the caries free group and this difference was highly statistically significant (p=0.012) [Table/Fig-2]. The median Candida albicans in the Subgroup B was numerically greater than Subgroup A in both SECC and caries free groups; but this difference was not statistically significant in both the groups [Table/Fig-3].
Observation for colony count of Candida albicans of all the samples.
| No. | SECC Group | Caries Free Group |
|---|
| A | B | A | B |
|---|
| 1 | 1.6 x 104 | 1.69 x 105 | 8 x 103 | 1.8 x 104 |
| 2 | 7 x 103 | 2.5 x 104 | 1 x 103 | 3.4 x 104 |
| 3 | 2.17 x 105 | 6.5 x 104 | 1 x 103 | 2.5 x 104 |
| 4 | 1.39 x 105 | 3.4 x 104 | 1 x 103 | 2 x 103 |
| 5 | 7.7 x 104 | 2.23 x 105 | 1 x 104 | 5 x 103 |
| 6 | 4.8 x 105 | 2.96 x 105 | 2 x 103 | 3 x 103 |
| 7 | 1 x 105 | 2.37 x 105 | 1 x 103 | 2.4 x 104 |
| 8 | 2 x 103 | 9 x 103 | 2.45 x 105 | 1 x 104 |
| 9 | 2 x 103 | 7 x 103 | 6 x 103 | 1 x 104 |
| 10 | 3.3 x 104 | 1 x 103 | 1.67 x 105 | 1 x 103 |
Chromagar for the detection of Candida albicans in SECC and caries free sample (respectively).
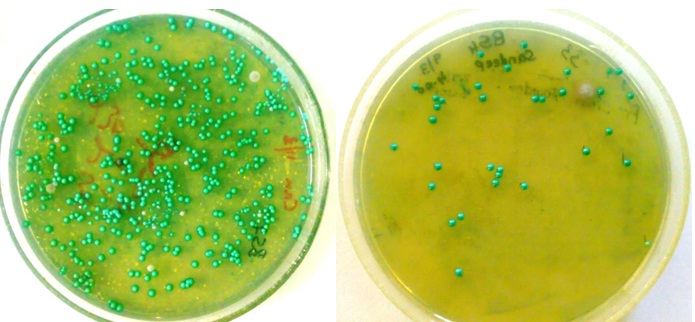
Candida albicans count in SECC and caries free groups.
| Group | N | MedianCFU/ml | IQR | Min | Max | MeanRank | Z | p-value |
|---|
| SECC | 20 | 3.35 x 104 | 1.975 x 105 | 1x 103 | 4.8 x 105 | 25.15 | -2.523 | 0.012** |
| Caries Free | 20 | 7 x 103 | 2.125 x 104 | 1 x 103 | 2.45x 105 | 15.85 |
**- Highly significant
IQR - Inter-quartile range
Z - Z-test
p - Probability
Tests done - Median, Minimum, Maximum Mean Rank, Probability.
Candida albicans count in the subgroups of both the SECC and caries free groups.
| Group | Sub- Group | N | MedianCFU/ml | IQR | Min | Max | MeanRank | Z | p-value |
|---|
| SECC | A | 10 | 2.45 x 104 | 1.5 x 105 | 2.0 x 103 | 4.8 x 105 | 9.75 | -0.567 | 0.570NS |
| B | 10 | 4.95 x 104 | 2.2 x 105 | 1.0 x 103 | 2.96 x 105 | 11.25 |
| Caries Free | A | 10 | 4.0 x 104 | 4.8 x 104 | 1.0 x 103 | 2.45 x 105 | 9.15 | -1.030 | 0.303NS |
| B | 10 | 1.0 x 104 | 2.15 x 104 | 1.0 x 103 | 3.4 x 104 | 11.85 |
NS- Not significant
Z - Z-test
p - Probability
IQR- Inter-quartile range
Tests done - Median, Minimum, Maximum Mean Rank, Probability
Comparison of the median Candida albicans count between the study subgroup and the control subgroup (i.e., SECC Subgroup A and caries free Subgroup A and between SECC Subgroup B and caries free Subgroup B) within the subgroups based on age revealed that Candida albicans count was numerically higher in the study subgroup compared to the control; but this difference was statistically significant only between the Subgroup B (p=0.045).
Discussion
Early childhood caries is an infectious disease of bacterial origin. The disease is the result of frequent sugar intake leading to changes in the oral microbial ecology to a cariogenic microflora, leading to an imbalance between the demineralization and remineralization process, favoring demineralization of the teeth. Thus, acidity is a pre-requisite for caries formation, and acidogenic microflora plays an important role [12].
SECC is a destructive form of dental caries affecting very young children. A number of studies have been reported regarding its prevalence, etiopathogenesis, prevention and management; but still the prevention and management of this disease remains a challenge to the dental practitioner [13]. Streptococcus mutans and Lactobacillus species are the main microorganism responsible for the initiation and progression of caries respectively. Actinomyces, Bacteroids, Bifdobacterium, Campylobacter, Capnocytophaga, Cornybacteria, Fusobacteria, Neisseria, Prevotella, Selenomonas, Veillonella species, Propionibacterium, Atopobium and other low pH non-streptococci are the other microorganisms implicated in caries pathogenesis. Currently researchers have questioned the role of Candida in ECC [14].
There is an ability of Candida albicans and Streptococcus mutans together to form biofilm which is enhanced in vitro and in vivo. The Candida albicans helps in the production of Exopolysaccharides (EPS), such that co-species biofilms will receive more biomass and harbor more viable Streptococcus mutans cells in increasing increments than single-species biofilms. The three dimensional biofilm architecture which is formed as a result of that displays sizeable Streptococcus mutans microcolonies surrounded by fungal cells, which are enmeshed in a dense EPS-rich matrix. The samples which were simultaneously infected by both species showed higher levels of infection and microbial carriage within plaque biofilms than those infected with either species alone. Furthermore, simultaneously infected EPS-rich matrix synergistically enhanced biofilm virulence, leading to aggressive onset of the rampant carious disease lesions [15]. Studies revealed that glucosyltransferase-derived EPS is a key mediator of co-species biofilm development and that coexistence with Candida albicans causes the virulence genes in Streptococcus mutans (e.g., gtfB, fabM) to express itself. Studies have shown that fungal mannan and β-glucan provide sites for gtfB binding and gtfB activity. Candida-derived β1, 3-glucans contribute to the EPS matrix structure. The severity of a ubiquitous infectious disease in a clinically relevant site occurs by a novel mutualistic bacterium-fungus relationship [15].
Candida species are fungi which are common inhabitants of the normal oral microbiota found in infants. Candida is an opportunistic pathogen and in immunocompromised individuals it has the ability to cause a variety of infections. For instance till date, oral thrush in infants and chronic atrophic candidiasis (denture induced stomatitis) in adult are the known most common clinical manifestations of oral candidiasis. Candida albicans is the most prevalent Candida species in the oral cavity. Studies suggest the presence of Candida albicans in saliva, dental plaque and also infected dentin of children with early childhood caries [9,10].
M. Sonesson et al., suggested that children of age below four years are under the state of developing immunity; and Candida spp. has potential to increase in number during low immune states. This could be an important factor for the rapid caries progression in children of this age group [7]. Candida albicans can exist in three morphotypes namely budding yeast, pseudohypha, and true hypha and hence, is called a polymorphic fungus. The morphological change from yeast to hyphal cells is important for its virulence and biofilm formation. Studies have found the isolation of yeast to be in proportion with increase in number of carious teeth, and reported an association between dental caries and the presence of yeast in the oral cavity.
Candida albicans is considered a normal commensal of the oral cavity, yet studies have reported Candida carriage to range from 23.7% - 89% in ECC and 7%-21% in caries free children [5,16–18]. Higher prevalence of Candida was also found in children using pacifiers. Its presence in the oral cavity may be related to many factors such as baby’s feeding bottles, infected pacifiers, infection at birth, nurse fingers, hospital maternity ward, maternal skin, air, water and carious teeth. However, our study showed 100% Candida prevalence from saliva of caries and caries free children [Table/Fig-1]. Our finding is also contradictory to that of Thaweboon et al., who reported an absence of Candida in caries free group [19,20].
Candida albicans count was found to be significantly higher in SECC group as compared to the caries free group [Table/Fig-2]. This finding is similar to that of many studies which have found the isolation of yeast to be in proportion with increase in number of carious teeth, and suggested a possible association between presence of yeast in the oral cavity and dental caries [3,6,21–23], but was in contradiction to Fabiola Galbiatti de Carvalhoa who found Candida levels in caries free group to be higher when compared to the SECC group [24]. The severity of the disease in SECC could be attributed to the greater role played by Candida albicans with its greater acidogenic potential. Role of Candida albicans in SECC needs further exploration for better understanding the etiopathogenesis of SECC.
Another assumption in this study was that since children below three years of age are under the state of developing immunity, they are at a higher risk for opportunistic infection namely Candida. Considering the acidogenic potential of Candida it could be the probable reason for the severity of the disease progression during early childhood. However, our finding was contradictory to the above assumption, i.e., Candida albicans count was lower in 12-36 months old children in both the SECC group and caries free group than 37-71 months old children and also absence of a significant difference in colony counts between the SECC Subgroup and caries free Subgroup in the 12-36 months old children (Group A).
Limitation
This article emphasizes on Candida albicans association with the caries process. The study has some limitations. The sample size was small so we cannot extrapolate these data to all individuals and all situations. It still remains unclear whether Candida species are causative agents in early childhood caries initiation or progression, or whether Candida colonization is merely a consequence of severe early childhood caries activity. Further research is required to elucidate the real role of this microorganism in the etiology of dental caries, which may help in management and prevention of this disease.
Conclusion
A high association was found between Candida albicans and SECC in this study. This study suggests that Candida albicans may be responsible for initiation and progression of SECC in the very young children with immature immune system.
Mouth rinses are used generally for their analgesic, anti-inflammatory, antimicrobial and anticariogenic activities to decrease the prevalence of dental caries in addition to the usual prophylactic measures like brushing and flossing. In addition to this anti-fungal agents should also be used so that the growth of Candida albicans (a fungus) is under control to prevent any dental caries caused due to it. Further studies are required in this area to help in management and prevention of this chronic childhood disease.
**- Highly significant
IQR - Inter-quartile range
Z - Z-test
p - Probability
Tests done - Median, Minimum, Maximum Mean Rank, Probability.
NS- Not significant
Z - Z-test
p - Probability
IQR- Inter-quartile range
Tests done - Median, Minimum, Maximum Mean Rank, Probability