Renal Biopsy Findings in Patients with Hypothyroidism: Report of 16 cases
Usha Singh1, Varnika Rai2, Rajeev Singh3, Deepa Santosh4, Jai Parkash5, Rana Gopal Singh6, Shivendra Singh7
1 Professor, Department of Pathology, Incharge UGC Advanced Immunodiagnostic Training and Research Centre, IMS BHU, Varanasi, Uttar Pradesh, India.
2 Junior Resident, Department of Pathology, IMS BHU, Varanasi, Uttar Pradesh, India.
3 Senior Resident, Department of Radiodiagnosis, IMS BHU, Varanasi, Uttar Pradesh, India.
4 Senior Resident, Department of Pathology, IMS BHU, Varanasi, Uttar Pradesh, India.
5 Professor, Department of Nephrology, IMS BHU, Varanasi, Uttar Pradesh, India.
6 Professor, Department of Nephrology, IMS BHU, Varanasi, Uttar Pradesh, India.
7 Associate Professor, Department of Nephrology, IMS BHU, Varanasi, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Varnika Rai, Sadhana Sadan, N4/35C-92, Mahamana Nagar Colony, Karundi, BHU, Varanasi-221005, Uttar Pradesh, India.
E-mail: vrachievers@gmail.com
Introduction
Hypothyroidism is prevalent in India. Its association with renal diseases though not very common but have been described in many studies. Here we are reporting renal biopsy findings in 16 cases, all of whom were already diagnosed cases of hypothyroidism.
Aim
To study renal parenchymal diseases associated in patients with hypothyroidism.
Materials and Methods
Formalin fixed paraffin embedded sections of renal biopsy were examined after staining with H&E, PAS and Acid Fuschin Orange G (AFOG) stain. Serum urea/creatinine measurements done by semi-autoanalysers and urine analysis were done by using urine strips and light microscopy.
Results
In 16 cases, M:F ratio was 9:7. Duration of disease varied from 6 months to 14 years. Blood urea and serum creatinine were raised in 10 cases (62.5%) and nephrotic range proteinuria was present in 13 cases (81.25%). Two of the patients had co existing systemic lupus erythaematous. Renal pathology revealed membranous glomerulonephritis (GN) in both cases.
In renal biopsy seven cases (43.75%) had pure Membranous Glomerulonephritis (MGN), 4 cases (25%) had mixture of Mesan-gial cell proliferation and membranous Glomerulonephritis(GN) also called MembranoProliferative GN (MPGN). Another four cases (25%) had Focal Segmental Glomerulosclerosis (FSGS) with chronic interstitial nephritis and one case was having minimal change disease.
Conclusion
Thus present study concludes that hypothyroidism can cause renal parenchymal disease like membranous GN, mesangiocapillary GN which is also called as membranoproliferative GN and FSGS.
FSGS, Membranous GN, Mixed GN, Mesangiocapillary GN, Minimal Change GN, Nephrotic Syndrome, SLE
Introduction
There is high prevalence of hypothyroidism in India affecting approximately one in ten people [1]. Thyroid disorders affect various body systems including renal function as well. Thyroid hormone is essential for adequate growth and development of kidney and maintains water and electrolyte homeostasis. Kidney in return plays a crucial role in metabolism and elimination of thyroid hormone [2]. Renal involvement has been found in primary and autoimmune hypothyroidism [3–6]. On the other hand non autoimmune hypothyroidism is more common in patients with proteinuria [7]. Various Renal function studies in hypothyroidism have shown significantly raised urea and serum creatinine and uric acid in overt hypothyroid subject whereas only significant rise in serum creatinine in subclinical hypothyroid, further strengthening the association between the hypothyroidism and renal function disorder [8].
Various types of glomerulonephritis have been reported in hypothyroidism e.g., IgA Nephropathy (IgAN), Mesangiocapillary GN (Mes Cap GN) also called membranoproliferative GN, Minimal change GN and Membranous GN [2,4,5,9,10]. Though aetiology of these GNs is not very clear it is supposed to be mainly due to circulating Immune Complexes (CIC) as Thyroglobulin or thyroid peroxidases antigen have been found deposited in kidney and thyroid tissue [11,12]. Autoimmune hypothyroidism often co exists with other autoimmune diseases like Systemic Lupus Erythaematous (SLE) and Rheumatoid Arthritis (RA) and type I diabetes mellitus, which may also lead to renal damage [13,14].
The present study was conducted on hypothyroid patients to find the association with renal involvement in cases of hypothyroidism.
Material and Methods
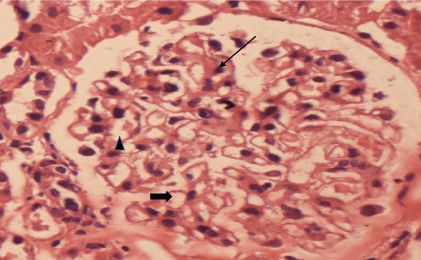
In the present retrospective study, out of total 205 cases of renal biopsies seen over a period of two years (June 2012 to July 2014), renal biopsies of 16 cases were described, who were already known cases of hypothyroidrism prior to emergence of renal disorders. All the cases were referred from Department of Nephrology of SS Hospital, Banaras Hindu University. Informed consent was taken from every patient included in the study. Clinical details, biochemical and immunological findings were recorded. Biochemical tests were done by semi-autoanalysers and urine analysis was done by using urine strips and light microscopy. Renal biopsies were fixed in 10% buffered formalin. Three micron thick sections were stained with Haematoxylin and Eosin (H&E) and Periodic Acid Schiff (PAS) stain. Acid Fuschin Orange G (AFOG) stains were done to see deposits in kidney biopsies. All these stains were done as per technique of Zollinger and Mihtasch 1978 [15]. Normal glomerulus with H&E staining is shown in [Table/Fig-1].
Normal glomerulous showing 1 -2 mesangial cells (arrow) per capillary tuft, open capillary channels(solid arrow), thin capillary wall(arrowhead) (H&E, 400X).
Results
Epidemiological and clinical findings in 16 patients have been mentioned in [Table/Fig-2]. All cases were suffering from hypothyroidism for more than 1 year & renal disease were detected later. All 16 cases had classical features of hypothyroidism in the form of dullness, coarse skin, cold intolerance, hoarseness of voice and lethargy.
Epidemiological and clinical findings in 16 patients. MGN: Membranous glomerulonephritis; MPGN: Membranoproliferative glomerulonephritis; FSGS: Focal segmental glomerulosclerosis; MCD: minimal change disease.
| No. of cases | 16 |
| M:F | 9:7 |
| Age range | Males- 27 to 60 yrs Females-25 to 48 yrs |
| Duration of illness | 4 months to 14 yrs |
| Clinical presentation | Proteinuria -13patients(81.3%);Raised urea/creat-10patients(62.5%);Microscopic haematuria 12 patients(75%). |
| Renal biopsy findings | MGN 7; MPGN 4; FSGS 4;MCD 1 |
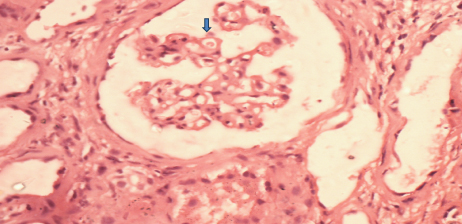
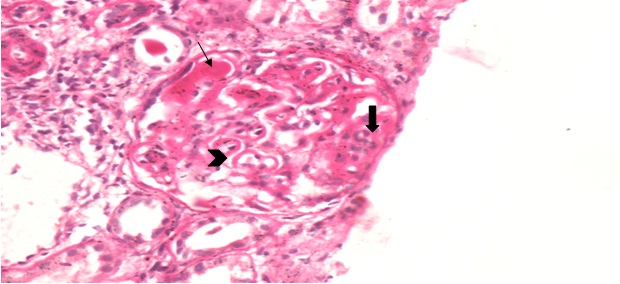
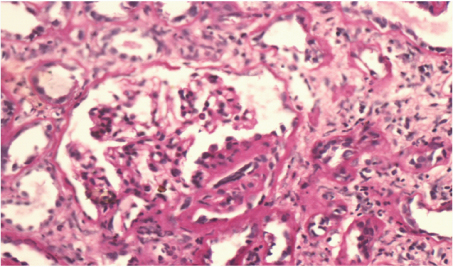
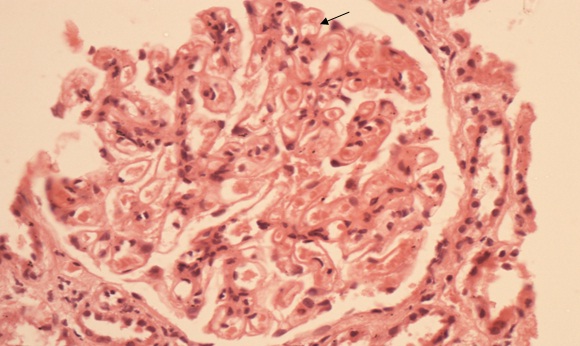
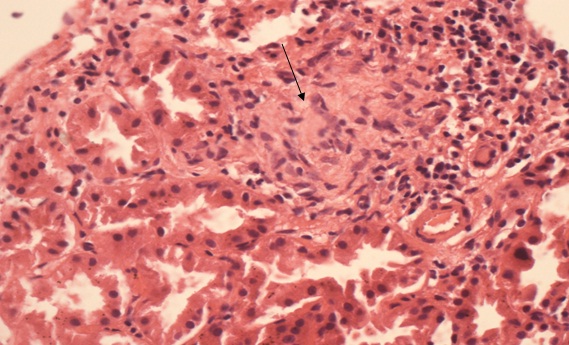
In renal biopsy seven cases (43.75%) had pure membranous GN (MGN) [Table/Fig-3], four cases (25%) had mixture of mesangial cell proliferation and membranous GN also called membranoproliferative GN (MPGN) [Table/Fig-4]. Another four cases (25%) had Focal Segmental Glomerulosclerosis (FSGS) with chronic interstitial nephritis [Table/Fig-5] and one case was having minimal change disease [Table/Fig-6].
Uniform thickening of GBM (thick arrow) without any cellular proliferation (H&E X 400).
Membranous thickening in half of the segment(arrow head) and mesangial cell proliferation, increase in mesangial matrix GBM thickening (solid arrow) and hyaline thrombi in capillaries in rest half of glomerulous (arrow) (PAS stain X 400).
Focal segmental glomerulosclerosis in right glomerular segment. Tubules show patchy atrophy. Interstitium shows mixed mononuclear cells and neutrophils infiltration focal fibrosis.(PAS X 400).
Normal glomerulous surrounded by dense mononuclear cell infiltration in the the interstitium and focal tubular atrophy and was diagnosed as minimal change GN with chronic interstitial nephritis.(PAS, 400X).
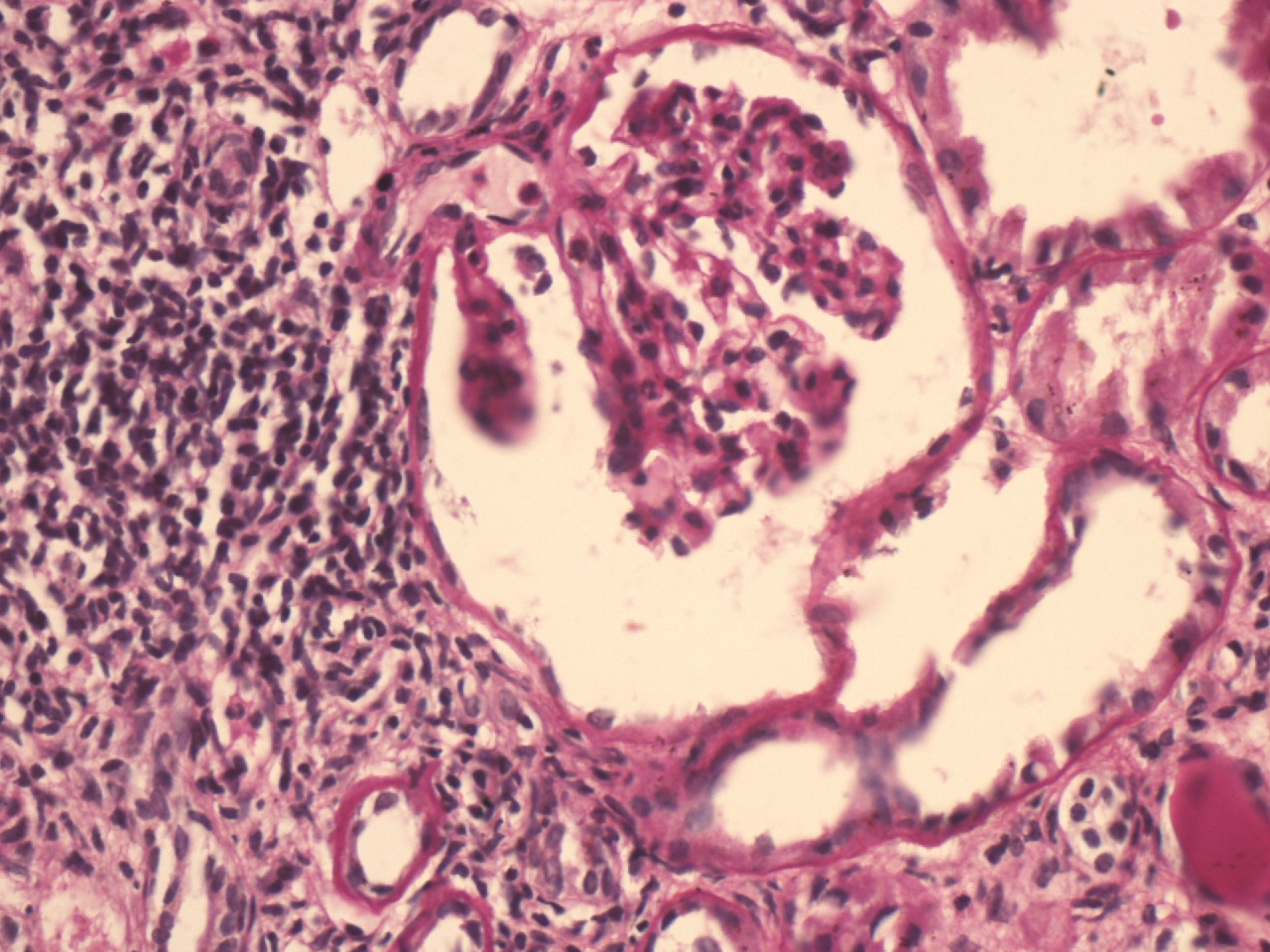
Our two cases (12.5%) had SLE and antithyroid peroxidase antibodies were positive in both of them. In one case it was diagnosed three years back, in another it was diagnosed five years back. Both cases had typical membranous GN with venulitis and chronic interstitial nephritis [Table/Fig-7,8]. Thus two cases of membranous GN (28.57%) were associated with lupus nephritis as well as autoimmune thyroiditis.
Membranous thickening of GBM (arrow) (H&E X 400).
Showing vasculitis of small blood vessels along with patchy mononuclear cell infiltration (H&E X 400).
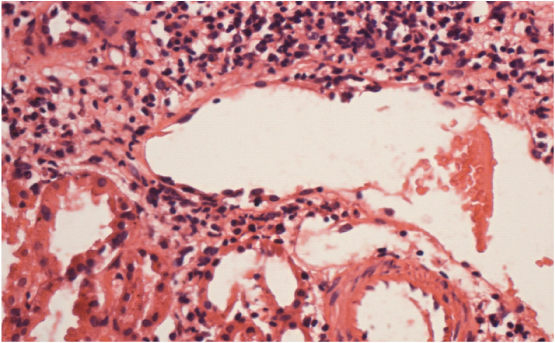
Two of our patients of FSGS (50%) had pulmonary tuberculosis for which they were taking antitubercular treatment. One of these patients showed granulomas in interstitium [Table/Fig-9].
Interstitium is showing epitheloid non caseating granuloma (arrow) along with mononuclear cells (H&E X 400).
In mixed mesangiocapillary GN, majority of glomeruli showed mixture of typical mesangiocapillary GN and dominant membranous GN. In AFOG stain, focal subepithelial and subendothelial deposits were seen. After one month follow up, all patients were alive and stable.
Discussion
In our study, no sex predominance was found. Nephrotic syndrome is the most common manifestation seen in hypothyroid associated renal diseases [9,16–20] In our study also, 81.25% patients presented with nephrotic syndrome.
In histopathology, membranous GN (MGN) was predominant finding. In 43.75% cases it was typical MGN. While in four cases it was predominantly membranous GN admixed with mesangial and endothelial proliferation and labeled as mixed GN or mesangiocapillary GN. Like us several other studies also reported predominance of membranous GN in hypothyroidism [21].
Other renal diseases in hypothyroidism are minimal change disease, membranoproliferative GN and IgA nephropathy [4,5,16,18–20]. There was one case of miniml change GN and 4 cases of FSGS with chronic interstitial nephritis in our study.
Renal pathology in four cases was found to be FSGS with chronic interstitial nephritis. Two of the patients of FSGS had pleural effusion for which they were taking antitubercular treatment. All though all four patients of FSGS had tubulointerstitial nephritis but in one case which was on ATT epitheloid cell granuloma was also present. Reports on interstitial nephritis and FSGS are sparse [22], however Dargan et al., in 2012 described hypothyroidism in five children in age group of 3 to 11 years, followed by 5 to 42 months [7]. These children developed hypothyroidism but there was no evidence of autoimmunity. All cases were resistant to all therapy and showed deterioration of renal functions in 1.5 to 14.5 years. Some studies have reported that hypothyroidism is side effect of multidrug resistance tuberculosis treatment. Ethionamide has related action to prothionamide which has similar actions to propyl thiouracil and methimazole and inhibit thyroid hormone synthesis by inhibiting iodine organification. Recent study done by Satti et al., 2012 found very high rate of hypothyroidism in cohort of MDR-TB patient [23]. In 69% cases, hypothyroidism occurs 93 days after starting antitubercular treatment [combination of PAS, cycloserine, pyrazinamide and ethionamide] our cases probably were due to antitubercular treatment. Other possibility is that tuberculosis have produced FSGS and interstitial nephritis and hypothyroidism both [23].
In present study, two (12.5%) cases with history of SLE for five and three years were also seen. In former hypothyroidism was detected 3years back and in later it was detected two years back. Both had membranous GN with chronic interstitial nephritis and vasculitis and both were female. In both cases antithyroid peroxidase antibodies were positive. Autoimmune hypothyroidism has been reported in 5.7 to 17% patients of SLE in different series [13,14,24].
Limitation
Immunofluroscence and electronmicroscopy study should have been done for better categorization and understanding. Due to economical and technical constraint we were not able to perform them.
Conclusion
In present study various types of glomerulonephritis were found associated with hypothyroidism. Membranous glomerulonephritis was the most common type of glomerulonephritis found in hypothyroidism associated renal parenchymal diseases.
[1]. Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N, Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of IndiaIndian J Endocr Metab 2013 17:647-52. [Google Scholar]
[2]. Iglesias P, Díez JJ, Thyroid dysfunction and kidney diseaseEur J Endocrinol 2009 160(4):503-15. [Google Scholar]
[3]. Gilles R, den Heijer M, Ross AH, Sweep FC, Hermus AR, Wetzels JF, Thyroid function in patients with proteinuriaNeth J Med 2008 66(11):483-85. [Google Scholar]
[4]. Iwazu Y, Nemoto J, Okuda K, Nakazawa E, Hashimoto A, Fujio Y, A case of minimal change nephrotic syndrome with acute renal failure complicating Hashimotoâs diseaseClin Nephrol 2008 69(1):47-52. [Google Scholar]
[5]. Uddin MJ, Alam KM, Mohammed FR, Alam MB, Hypothyroidism and Nephrotic Syndrome- A Rare AssociationJ Medicine 2009 10:34-55. [Google Scholar]
[6]. Basu G, Mohapatra A, Interactions between thyroid disorders and kidney diseaseIndian J Endocrinol Metab 2012 16(2):204-13. [Google Scholar]
[7]. Dagan A, Cleper R, Krause I, Blumenthal D, Davidovits M, Hypothyroidism in children with steroid-resistant nephrotic syndromeNephrol Dial Transplant 2012 27(6):2171-75. [Google Scholar]
[8]. Saini V, Yadav A, Arora MK, Arora S, Singh R, Bhattacharjee J, Correlation of creatinine with TSH levels in overt hypothyroidism - a requirement for monitoring of renal function in hypothyroid patients?Clin Biochem 2012 45(3):212-14. [Google Scholar]
[9]. Paydas S, Gokel Y, Different renal pathologies associated with hypothyroidismRen Fail 2002 24(5):595-600. [Google Scholar]
[10]. Dizdar O, Kahraman S, Genctoy G, Ertoy D, Arici M, Altun B, Membranoproliferative glomerulonephritis associated with type 1 diabetes mellitus and Hashimoto’s thyroiditisNephrol Dial Transplant 2004 19:988-89. [Google Scholar]
[11]. Shima Y, Nakanishi K, Togawa H, Obana M, Sako M, Miyawaki M, Membranous nephropathy associated with thyroid-peroxidase antigenPediatr Nephrol 2009 24(3):605-08. [Google Scholar]
[12]. Brohee D, Delespesse G, Debisschop MJ, Bonnyns M, Circulating immune complexes in various thyroid diseasesClin ExpImmunol 1979 36(3):379-83. [Google Scholar]
[13]. Pyne D, Isenberg DA, Autoimmune thyroid disease in systemic lupus erythaematosusAnn Rheum Dis 2002 61(1):70-72. [Google Scholar]
[14]. Porkodi R, Ramesh S, Mahesh A, Kanakarani P, Rukmangathrajan S, Panchapakesa C, Thyroid dysfunction in systemic lupus erythaematosus and rheumatoid arthritisJ Indian Rheumatol Assoc 2004 12:88-90. [Google Scholar]
[15]. Zollinger HU, Mihatsch MJ, Renal Pathology in Biopsy Light, electron and immunofluorescent microscopy and clinical aspects 1978 2nd editionBerlin Heidelberg, New YorkSpringer-Verlag:8-20. [Google Scholar]
[16]. Gurkan S, Dikman S, Saland MJ, A case of autoimmune thyroiditis and membranoproliferative glomerulonephritisPediatr Nephrol 2009 24(1):193-97. [Google Scholar]
[17]. Wu MT, Huang SC, Chen YL, Lee CT, Concurrent hypothyroidism and IgA nephropathy presenting with acute heart and oliguric renal failureActa Nephrology 2012 26:100-03. [Google Scholar]
[18]. Nishimoto A, Tomiyoshi Y, Sakemi T, Kanegae F, Nakamura M, Ikeda Y, Simultaneous occurrence of minimal change glomerular disease, sarcoidosis and Hashimoto’s thyroiditisAm J Nephrol 2000 20(5):425-28. [Google Scholar]
[19]. Deák G, Ruzicska E, Somogyi A, Association of IgA nephropathy, hypothyroidism and hypercholesterolemiaJ Nephrol 2005 18(6):773-76. [Google Scholar]
[20]. Enríquez R, Sirvent AE, Amorós F, Andrada E, Cabezuelo JB, Reyes A, IgA nephropathy and autoimmune thyroiditisClin Nephrol 2002 57(5):406-07. [Google Scholar]
[21]. Grcevska L, Polenakovic M, Petrusevska G, Membranous nephropathy associated with thyroid disordersNephron 2000 86(4):534-35. [Google Scholar]
[22]. Yamada S, Bandai S, Masutani K, Tsuchimoto A, Noguchi H, Munakata M, Focal segmental glomerulosclerosis in a patient with isolated ACTH deficiency and reversible hypothyroidismClin Exp Nephrol 2010 14(2):168-72. [Google Scholar]
[23]. Satti H, Mafukidze A, Jooste PL, McLaughlin MM, Farmer PE, Seung KJ, High rate of hypothyroidism among patients treated for multidrug-resistant tuberculosis in LesothoThe International Journal of Tuberculosis and Lung Disease 2012 16(4):468-72. [Google Scholar]
[24]. Kakehasi AM, Dias VN, Duarte JE, Thyroid abnormalities in systemic lupus erythaematosus: a study in 100 Brazilian patientsRev Bras Reumatol 2006 46(6):375-79. [Google Scholar]