The success of root canal treatment can be achieved by a thorough biomechanical preparation followed by the complete filling of the prepared root canal so that bacteria can’t enter the root canal through the oral cavity or periapex [1]. Most of obturating systems in endodontic therapy have a core material and a sealer. The sealer should not only fill the gaps between the core material and the root canal wall, but also should fill the minor irregularities on the surface of the prepared root canal to make a fluid tight seal [2].
There is presence of bacteria in the dentinal tubules and cementum even after the treatment has been reported [3], which means the goals of total disinfection of the canal may not be achieved by cleaning and shaping only [4].
One of the ideal requirements of root canal sealers is its antibacterial nature, so that it can eradicate the remaining bacteria [5]. Antibacterial nature of sealer is much valuable because of the higher percentage of facultative anaerobes in failed root canal cases [1].
Ideal sealer should be able to kill the remaining bacteria present on the dentinal walls of root canals along with those present deep inside the dentinal tubules. To achieve this, the sealer should not only kill the bacteria by contact action, but also should be able to diffuse inside the dentinal tubules. This is possible only if the sealer has good flow properties. So while planning to measure antibacterial properties of a sealer, the contact and diffusibility of the sealer should be taken into consideration along with its flow property.
There are many root canal sealers available in the market that claim to have an antimicrobial activity against microorganisms present in the root canal. They are based on different formulations like Mineral Trioxide Aggregate (MTA), calcium hydroxide, resin and zinc oxide. Therefore the purpose of this study was to compare invitro antimicrobial activity and flow characteristics of four endodontic sealers; Epoxy resin based (AH Plus), MTA based (MTA Fillapex), Calcium hydroxide based (Calcibiotic Root Canal Sealer-CRCS), powdered gutta-percha (Gutta Flow 2) on Enterococcus faecalis.
Materials and Methods
This study was conducted at Department of Conservative Dentistry and Endodontics in collaboration with Department of Microbiology, King George’s Medical University Lucknow, Uttar Pradesh, India, in January 2016.
In this invitro observational study, 4 root canal sealers AH-Plus (Dentsply de Trey, Konstanz, Germany), CRCS (Coltene/Whaledent Inc. USA), MTA Fillapex (Angelus, Londrina, Brazil) and Gutta Flow 2 (Coltene/Whaledent Germany) were tested and compared for their antimicrobial activity against E. faecalis ATCC 29212 whereas bacteriologically sterile normal saline was taken as negative control. All sealers were selected on the basis of their different chemical formula i.e., Resin based, calcium hydroxide, calcium silicate, and powdered gutta-percha. The sealers were prepared in accordance with the manufacturer’s recommendations.
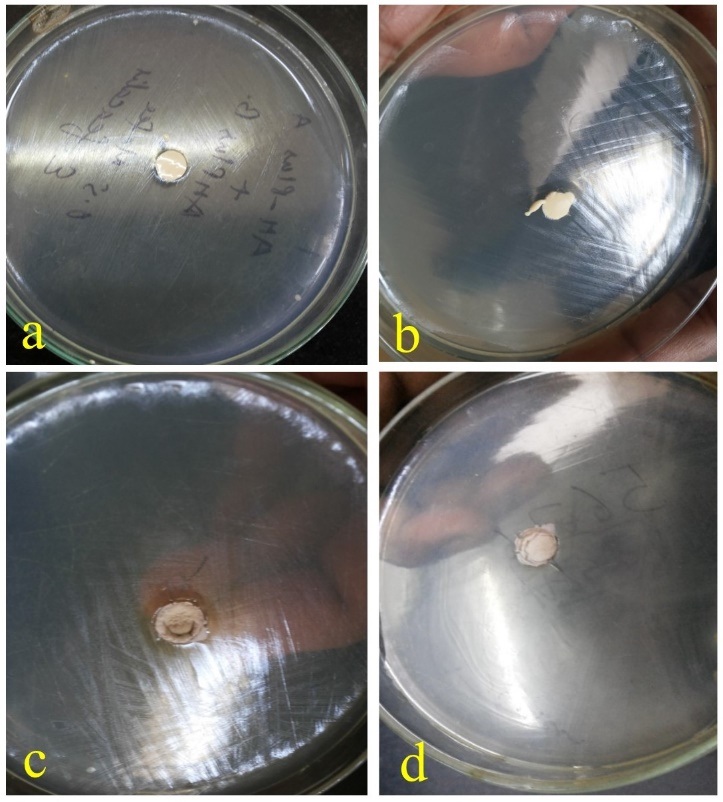
Agar Diffusion Test (ADT) was done using Muller Hinton agar (Himedia Laboratories, Mumbai, India). The test was done under strict aseptic conditions in class II, type A2 biological safety cabinet. All the 4 sealers were placed in the wells of 4mm depth and 6mm diameter punched in 10 plates of Muller Hinton agar. The surface of each agar plate was inoculated by swabbing with 0.5 ml McFarland standard suspension of E. faecalis ATCC 29212. These plates were incubated at 37oC for seven days. The diameters of the zones of inhibition around each well were measured in millimetres (mm) at 24 hours [Table/Fig-1a-d] and after the 7th day of incubation. The mean diameter of measured zone was determined for all the four sealers.
ADT of different sealers at 24 hours.
a-AH Plus, b- CRCS, c- MTA Fillapex, d- Gutta Flow 2
Direct Contact Test (DCT) was done by settling 50mg of freshly mixed sealer in a sterile flat bottom screw capped tubes. Ten tubes were prepared for each sealer in duplicate. After that 50 μL of 0.5 ml McFarland standard suspension (1.5 x 108 CFU/ ml) of E. faecalis ATCC 29212 was pipetted and spread over the sealers. The test tubes were incubated at 37oC which ensured direct contact between bacteria and test sealers. These tubes were divided into two equal sub groups to be analysed at 1 hour and at 24 hours. The suspension of E. faecalis and test sealers were allowed to be in the contact for 1 hour and 24 hours for all the sealers. To determine the colony count of the suspension in both groups, the suspensions were diluted by adding 450μL of sterile nutrient broth to the screw-capped tubes. From each of these vials, 10 μL of suspension was drawn and spread over Mac Conkey agar to determine the colony count with a digital colony counter (Cole-Parmer, India). A suspension of E. faecalis ATCC 29212 without the sealer in the normal saline was taken as the control and sub cultured after 1 and 24 hours, and the colony count was determined. Colony counts of all the sealers in both the groups (1st and 24 hours) were also determined in a similar manner. Thus the immediate (after 1 hour) and delayed (after 24 hours) antimicrobial efficacy of all the materials against E. faecalis were evaluated. The data obtained was analysed by one way Analysis of variance (ANOVA). The paired t-test was used to compare the changes. The spearman correlation coefficient was calculated in ADT among the groups. The p-value<.05 was considered significant.
Flow test was carried out in accordance with ADA specification No. 57. A well and thoroughly mixed mass of sealer was prepared according to manufacturer’s instruction. A 0.5 ml of this mix was dropped on the centre of a clean glass slab and after three minutes another glass slab was placed on it with a weight making total mass of 120 grams. Sealer was spread in a circular disc pattern. After 10 minutes of initial mixing maximum and minimum diameter of the circular disc of sealer was measured by digital callipers in millimetre (mm).
Validation of test was done by two mandatory conditions otherwise test was repeated;
The difference between maximum and minimum diameter was not more than 1.0 mm.
The circular pattern had uniform thickness which was assessed visually.
For each sealer, the experiment was repeated for five times. The diameter of the sealer was measured at five different points each time [6]. Obtained data, then analysed by one-way ANOVA. Pairwise comparison was done by Tukeys (5%) test and p-value <.05 was considered significant.
Results
ADT showed statistically significant difference in microbial inhibition among AH plus, CRCS and MTA Fillapex. A zone of inhibition at 24 hours was highest in CRCS (15.1±0.01) and lowest in AH Plus (4.15±0.02) [Table/Fig-2]. After seven days of incubation the zone of inhibition decreased in AH plus, CRCS and MTA Fillapex but the difference was found to be statistically significant (p=0.0001). Gutta Flow 2 and control did not demonstrate any microbial inhibition [Table/Fig-2].
Shows results of agar diffusion test and direct contact test at different time intervals.
| Groups | ADT (in mm) | DCT (no of organisms/ml) |
|---|
| 24 hour | 7 days | 1st hour | 24 hours |
|---|
| Control | 0 | 0 | 1.5×108 | 1.5×108 |
| AH Plus | 4.15± .02 | 2.15±0.23 | 5.7 ×102 ± .02 | 3.64 ×102 ± .013 |
| CRCS | 15.1± .01 | 11.96±.35 | 6.2 ×102 ± .025 | 2.72 ×102 ± .015 |
| MTA Fillapex | 13.5± .01 | 11.12±.25 | 5.02 ×102 ± .01 | 2.73 ×102 ± .017 |
| Gutta Flow 2 | 0 | 0 | 0.15×108 | 0.14 ×108 |
| ANOVA p-value | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
* Statistically significant
One-way ANOVA was used to compare the groups at different intervals.
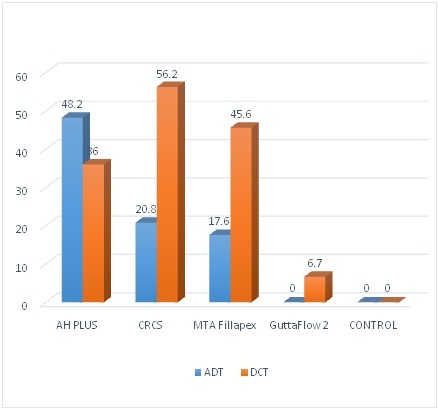
There was a significantly better correlation of bacterial inhibition growth between AH Plus and CRCS (r=0.81, p=0.0001) and MTA (r=0.72, p =0.0001) at 24 hours. The correlation was significant between CRCS and MTA (r=0.66, p=0.001) at 24 hours. The correlations were insignificant (p>.05) at 7 days among the groups [Table/Fig-3]. The reduction in bacterial inhibition was higher in AH plus (48.2%) group than CRCS (20.8%) and MTA (17.6%) from 24 hours to seven days and one-way ANOVA showed these changes was statistically significant [Table/Fig-2] (p<.001).
Spearman correlation of antibacterial activity of different sealers at 24 h and 7 days.
| | AH plus at 24 hour | CRCS at 24 hour | MTA Fillapex at 24 hour | AH plus at 7 days | CRCS at 7 days | MTA Fillapex at 7 days |
|---|
| AH plus at 24 h | r | 1.000 | | | | | |
| p-value | . | | | | | |
| CRCS at 24 h | r | 0.818** | 1.000 | | | | |
| p-value | 0.0001 | . | | | | |
| MTA Fillapex at 24 h | r | 0.723** | 0.666** | 1.000 | | | |
| p-value | 0.0001 | 0.001 | . | | | |
| AH Plus at 7 days | r | 0.948** | -0.248 | -0.116 | 1.000 | | |
| p-value | 0.0001 | 0.490 | 0.751 | . | | |
| CRCS at 7 days | r | 0.454 | 0.031 | -0.214 | 0.603 | 1.000 | |
| p-value | 0.188 | 0.933 | 0.552 | 0.065 | . | |
| MTA Fillapex at 7 days | r | -0.116 | -0.500 | 0.122 | -0.127 | -0.423 | 1.000 |
| p-value | 0.749 | 0.141 | 0.738 | 0.727 | 0.223 | . |
**. Correlation is significant at the .01 level (2-tailed)
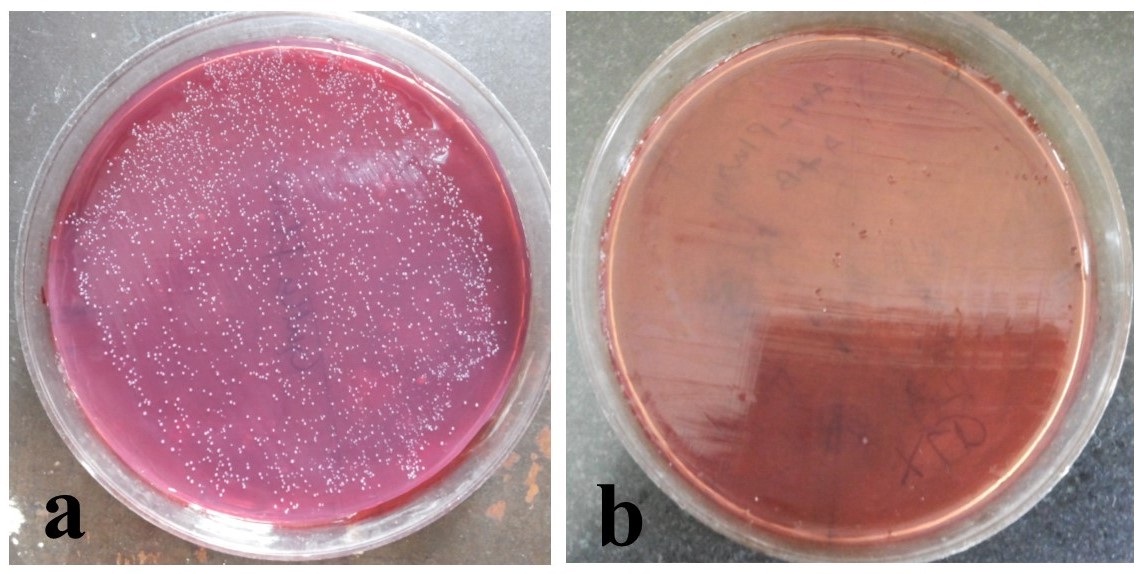
Direct Contact test: The direct contact test showed a significant difference in the number of organisms among the groups (ANOVA p=0.0001) at 1st hour and 24 hours [Table/Fig-4]. The post-hoc test showed a significant (p<0.01) lower number of organisms in AH Plus, CRCS and MTA than controls at 1st as well as at 24 hours [Table/Fig-2]. Gutta Flow 2 had a lower number of organisms than controls at both 1st and 24 hours [Table/Fig-2]. The average percent reduction was higher in Gutta Flow 2 (6.7%) than CRCS (56.12%), MTA (45.6%) and AH Plus (36%). There was no change in the number of organisms in controls [Table/Fig-5].
DCT of Control and AH Plus at 24 hours.
Growth of E. faecalis colonies shown on mac conkey agar at 24 hours in; a-control (sterile normal saline), and b- AH Plus.
Graph Shows average percent change in the bacterial inhibition (from 24 hour to 7 days) in agar diffusion test and number of organism (from 1st hour to 24 hours) in direct contact test among the groups. (p=0.0001).
One way analysis was used to test the significance of differencein antibacterial activity of sealers.
In this study, CRCS showed highest microbial inhibition followed by MTA Fillapex and AH Plus after 24 hours. Gutta Flow 2 did not show any inhibition after 24 hours and 7 days [Table/Fig-2].
Flow test: AH Plus showed the maximum flow, whereas CRCS showed the minimum flow. Mean diameter of disc formed by AH Plus was 40.44mm, 35.30mm for MTA Fillapex, 30.71mm for Gutta Flow 2 and 28.96 mm for CRCS [Table/Fig-6].
Flow of tested sealers (mm).
| Sealer | Flow (mean ± SD)One-way ANOVA (F= 18.68, p=0) |
|---|
| AH Plus | 40.4360 ±5.7462 |
| MTA Fillapex | 35.3060± 0.8058 |
| Gutta Flow 2 | 30.7140±1.0128 |
| CRCS | 28.9580± 2.1457 |
Flow of sealers differs significantly, it was maximum in AH Plus and minimum in CRCS.
On Pairwise comparison by post-hoc Tukey HSD, flow of AH Plus was significantly higher then Gutta Flow 2 and CRCS (p=0.001) while the difference of flow was insignificant between AHPlus and MTA Fillapex (p=0.084). Significant difference in flow also found between MTA Fillapex and CRCS (p= 0.026) [Table/Fig-6,7].
Pairwise comparison of flow by post-hoc Tukey HSD.
| Comparison | ‘p’ (95% confidence interval) |
|---|
| AH Plus vs MTA Fillapex | 0.084 (-0.54—10.80)Not Significant |
| AH Plus Vs Gutta Flow 2 | 0.001 (4.05—15.39)Significant |
| AH Plus Vs CRCS | 0 (5.80—17.14)Significant |
| MTA Fillapex Vs Gutta Flow 2 | 0.136 (-1.07—10.26)Not Significant |
| MTA Fillapex Vs CRCS | 0.026 (0.67—12.01)Significant |
| Gutta Flow 2 Vs CRCS | 0.81 (-3.91— 7.47)Not Significant |
Discussion
Good antimicrobial property and flow rate are two most desirable properties of an ideal root canal sealer. The purpose of the present study was to evaluate the antimicrobial activity and flow rate of AH plus, CRCS, MTA fillapex and Gutta Flow 2 sealers against E. faecalis.
E. faecalis, which is a gram positive, facultative anaerobic microbe, was chosen in the present study because it is the most common microbe associated with failed root canal treatment cases. It has the ability to survive alone or with other microorganisms in the root canal [7]. Several virulence factors of E. faecalis help it to survive into the root canal even after the root canal therapy [8]. It has the ability to penetrate the dentinal tubules and adhere to collagen in the presence of human serum [9]. It is commonly associated with persistent apical periodontitis and seems to be difficult to eradicate from the root canal. Evans et al., suggested that this may be due to microbe’s ability to regulate internal pH and proton pump [10]. Apart from that, it can also bear prolonged starvation [11]. These findings may justify its use as a test organism in this study.
The antibacterial activity of these sealers are measured by ADT and DCT. These are two most common tests to evaluate the antimicrobial activity of endodontic sealers. ADT depends on the solubility and physical properties of the antimicrobial component of the sealer [12]. This test is more suitable with the water soluble materials [13]. On the other hand, in DCT, the antibacterial activity is not dependent upon solubility and diffusibility of tested materials [14]. DCT qualitatively evaluates the antibacterial activity of the surface of the material with very low solubility, in this test bacteria are allowed to come in direct contact with the test material (Sealer) [15].
This study shows that the reduction in bacterial inhibition was higher in AH plus group than CRCS and MTA from 24 hours to seven days and the change was statistically significant [Table/Fig-5]. The correlation analysis showed that a significant correlation with antibacterial property was found in AH plus, CRCS and MTA Fillapex at 24 hours. However, no such significant association was observed at seven days. This shows that resin based sealers are more effective in fresh mixed state and their antibacterial activity reduces over time. Similar results were also reported in other studies [16,17].
In the present study, results of ADT show that CRCS makes a largest inhibition zone compared to MTA Fillapex, AH plus and Gutta Flow 2.
The hydroxyl ions of calcium hydroxide increase the pH of the site during its diffusion into the surrounding dentine. Increased pH at the site aids in repair and calcification. These qualities of calcium hydroxide make it the material of choice in selection of intracanal medicament [18]. Similar results are also reported by Cavalcanti A et al., [19].
MTA Fillapex shows second highest antibacterial potential after CRCS among all tested sealers. The main ingredient of MTA Fillapex is MTA, resins, Bismuth oxides, nanoparticulate silica, its pH after three hours was 9.68 than it decreased over time [20]. MTA based sealers not only stimulate mineralization [21], but also lay down apatite like crystals along the root canal wall [22]. The sealing ability of few MTA based sealers was found comparable to resin based sealers [23]. Prolonged exposure of calcium hydroxide to root canal dentine has a weakening effect on dentine and proposed that a short term application of calcium hydroxide followed by MTA could have prevented weakening of dentinal wall [24]. Its good radiopacity, better flow and high pH make it suitable root canal sealer but the literature supporting these findings are limited [25]. A study has shown that it has shorter setting time and lower water absorption than AH plus [26]. The elevated pH is the decisive factor for its antimicrobial action.
Epoxy resin based sealers like AH Plus has a good antibacterial effect, but this effect is best in freshly prepared sealer. This could be because of easy diffusion of antibacterial component in the surrounding environment before setting of the material [27]. Resin based sealers have an antibacterial effect due to presence of bisphenol A diglycidyl ether [28]. Slight suppression of growth of E. faecalis has been observed with a freshly mixed AH plus which also showed antibacterial activity against on F. nucleatum and P. gingivalis in the agar diffusion test [16].
Gutta Flow 2 did not show any significant bacterial reduction by any of the two methods. Gutta Flow 2 is an advancement of Gutta Flow which has the same composition as Gutta Flow (mixture of gutta-percha powder, poly-dimethylsiloxane and silver particles) but in different proportions [29].
The highest antimicrobial activity of CRCS, AH Plus, MTA Fillapex seen immediately after mixing which decreased over time. The set sealer did not release antimicrobial agent as much as unset sealer that’s why they showed a reduction in antibacterial activity after setting [Table/Fig-2].
Flow
As per ADA specification no. 57 the minimum flow of a root canal sealer should be ≥ 20 mm [6]. All the sealers tested in this study having flow above this value. Maximum flow observed presented by AH Plus and minimum value was observed with CRCS [Table/Fig-6]. Size of particle plays a vital role in flow characteristics of a sealer, it is inversely proportional to flow. If it is less then there are unfilled irregularities within the root canal wall and in between core material and root canal wall, higher flow rates may lead to extrusion of sealer from the apical foramina both the situations are undesirable for the success of root canal treatment. Hence, moderate flow of sealer is better [30].
To the best of our knowledge no previous study has compared these four sealers, this study was attempted to compare the antimicrobial activity and flow of the chemically different sealers. This invitro study was carried out on the ATCC 29212 strain of E.faecalis, study on patients strains are further required to validate our results.
Conclusion
Highest microbial inhibition was shown by CRCS, followed by MTA Fillapex, AH Plus and Gutta Flow 2. The antimicrobial activity of all sealers was reduced over time. Maximum reduction in antibacterial activity with time was seen with AH Plus. Considering flow properties, maximum flow was shown by AH Plus while minimum by CRCS.
* Statistically significant
One-way ANOVA was used to compare the groups at different intervals.
**. Correlation is significant at the .01 level (2-tailed)
Flow of sealers differs significantly, it was maximum in AH Plus and minimum in CRCS.