Antifungal Activity of Cinnamon Oil and Olive Oil against Candida Spp. Isolated from Blood Stream Infections
Nidhi Goel1, Hina Rohilla2, Gajender Singh3, Parul Punia4
1 Professor, Department of Microbiology, PGIMS, Rohtak, Haryana, India.
2 Senior Resident, Department of Microbiology, PGIMS, Rohtak, Haryana, India.
3 Senior Professor and Head of the Department, Department of Pharmacy, PGIMS, Rohtak, Haryana, India.
4 Senior Resident, Department of Microbiology, PGIMS, Rohtak, Haryana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nidhi Goe, Professor, Department of Microbiology, 2nd Floor, PGIMS ROHTAK, Haryana, India.
E-mail: ngoel_2003@yahoo.com
Introduction
Recently non-albicansCandida has emerged as a major cause of morbidity and mortality in blood stream infections. Some species of the Candida are becoming increasingly resistant to first line and second line antifungals such as echinocandins and fluconazole. In view of increasing global antifungal resistance, role of alternative and better antifungals like natural plant products need to be explored. Essential oils are known to exhibit antimicrobial activity against various fungi. Hence, we evaluated the efficacy of cinnamon oil and olive oil against Candida spp.
Aim
To evaluate the invitro antifungal activity of olive oil and cinnamon oil against blood stream Candida isolates.
Materials and Methods
The present prospective observational study was conducted in the Department of Microbiology at a tertiary care teaching hospital during one year June 2011-July 2012. Blood samples were collected from 1376 patients clinically suspected to have fungal septicaemia, out of which 100 (7.2%) Candida isolates obtained, were speciated by conventional methods. Antifungal susceptibility testing of all the isolates was done against fluconazole, voriconazole as per NCCL (M27-A2) and against olive oil and cinnamon oil by agar well diffusion method.
Results
Prevalence of Candidemia was 7.26%. C. albicans (85.3%) and C. parapsilosis (85.7%) were most sensitive to fluconazole followed by C. tropicalis (67.4%). All isolates were 100% sensitive to voriconazole. Both oils were found to be effective against nearly 50% of the Candida isolates. About 55.5% of fluconazole resistant C. krusei strains were sensitive to olive and cinnamon oil.
Conclusion
Fluconazole resistant non-albicansCandida has emerged as major cause of Candidemia. Cinnamon and olive oil show marked sensitivity against albicans and non-albicans spp.
Antifungal susceptibility, Candidemia, Essential oil, Fluconazole
Introduction
The dramatic rise in invasive fungal infections especially Candidemia has gained worldwide significance since last few decades. Compared to other microbial pathogens causing bloodstream infections, albicans and non- albicansCandida spp. are ranked fourth among the most common agents of bloodstream infections, and are associated with high mortality (30-40%) and morbidity [1]. There are mainly four major classes of antifungal drugs available to treat invasive fungal infections. They include polyenes, pyrimidine analogs, echinocandins, and triazoles. In medical practice, triazoles such as fluconazole and voriconazoles are still the most used antifungals. But, the use of antifungal drug therapy has led to the development of antifungal resistance to these common agents [1,2]. Hence, in view of increasing threats posed by drug resistant yeasts, we need to vigorously search for alternative and better antifungals, which are not only effective but also have lesser side effects. Traditional medicines have been known to play a major role in various health services. Various non antibiotic substances such as essential oils like olive oil and cinnamon oil have been reported to be effective against various yeasts and molds [3]. Essential oils are aromatic oily liquids which are obtained from various plant parts and are known to show wide spectrum of antimicrobial activity [3,4]. The use of these oils instead of antifungal drugs will be more preferable thus studies are needed to test the efficacy of these oils and hence with this purpose the present study was conducted.
Materials and Methods
The present prospective observational study was conducted in the Department of Microbiology in association with Department of Pharmacy during one year period from June 2011-July 2012. A total of 1376 patients of all age groups and sex visiting the OPD and admitted to the hospital with clinical suspicion of fungal septicaemia were included in the study. Patients who were already on antifungals were excluded from the study. Demographic and clinical data such as age, sex, birth weight, history of pre-term birth, antibiotic prophylaxis history, presence of any co-morbid condition and presence of any intravenous lines were noted for all these patients. Consecutive blood samples in duplicate were collected from each patient under aseptic precautions in two sets of biphasic Brain Heart Infusion (BHI) medium. One BHI set was incubated at 25°C and other at 37°C and subcultures were performed after 24 hours, 48 hours and 72 hours up to 7 days. The colonies were identified by standard microbiological techniques. A total of 100 Candida isolates were obtained and identified up-to the species level on the basis of germ tube test, morphology on cornmeal agar, growth on Hi-Chrome Candida agar, carbohydrate fermentation and sugar assimilation test [5,6].
Susceptibility testing
The isolated Candida isolates were further subjected to antifungal susceptibility testing against fluconazole (Hi-media), voriconazole (Pfizer), cinnamon oil and olive oil. Susceptibility testing for fluconazole and voriconazole was performed by the broth microdilution minimum inhibitory concentration (BMD-MIC) method using RPMI (Roswell Park Memorial Institute) medium and MOPS (3-(N-morpholino) propane sulphonic acid) buffer. MIC results were interpreted as per NCCL (M-27-A2) guidelines. The quality control test was performed using ATCC strain, C. albicans ATCC 90028, C. parapsilosis ATCC 22019, Candida tropicalisATCC750 and Candida krusei ATCC 6258 [7]. All the 100 isolates were subjected to susceptibility testing against cinnamon oil and olive oil by agar well diffusion method. Candida strain to be tested was inoculated on sterilized Sabouraud’s Dextrose Agar plate. A hole was punched aseptically by sterile cork borer of 6-mm diameter on the agar surface. A 50 μl of cinnamon and olive oil was introduced into each of the peripheral wells with sterilized DMSO as negative control. The plates were incubated at 28°C. Antimicrobial activity of essential oils was analysed by observing the zone of inhibition. The results were interpreted as <9 mm- inactive; 9-12 mm- partially active; 13-18 mm- active; >18 mm- very active [8,9]. Statistical analysis was performed by chi-square test using SPSS version- 16.0 software.
Results
During the study period, a total of 100 (7.26%) isolates of Candida spp. were isolated from 1376 patients. Rate of Candida isolation was higher in paediatric (1-15years) age group (89%) as compared to adults (> 15 years) (11%).
Majority of Candida strains were isolated from neonates (36%) followed by infants (28%), 1-15 years (25%) and adults (11%) [Table/Fig-1]. The male to female ratio was 3:1. The rate of Candida isolation was maximum from PICU (Paediatric Intensive Care Unit) patients (72%) followed by wards (21%) and OPD’s (7%) [Table/Fig-2].
Age wise distribution of various candida species.
| Age | Total(n=100) | C.albicans(n=41) | C.tropicalis(n=43) | C.krusei(n=9) | C. parapsilosis(n=7) |
|---|
| < 1 month | 36 | 19 (52.78%) | 10 (27.78%) | 4 (11.11%) | 3 (8.33%) |
| 1-12 months | 28 | 8 (28.6%) | 16 (57.14%) | 2 (7.14%) | 2 (7.14%) |
| 1-15 years | 25 | 11 (44%) | 12 (48%) | 1 (4%) | 1 (4%) |
| Adults | 11 | 3 (27.27%) | 5 (45.45%) | 2 (18.18%) | 1 (9.05%) |
Distribution of isolates according to clinical settings.
| Clinical Settings | Number of Candida isolates n=100 |
|---|
| PICU | 72 |
| Ward | 21 |
| OPD | 07 |
| Total | 100 |
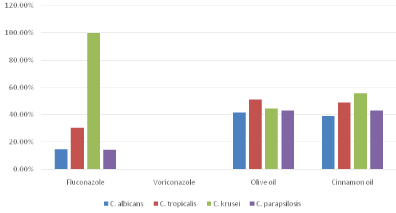
Out of the 100 Candida isolates, C. tropicalis (43%) was the most common isolate followed by C. albicans (41%), C. krusei (9%) and C. parapsilosis (7%). C. albicans was more frequently isolated from the infants (0-1month) whereas C. tropicalis was more common in the neonates (1-12 months) [Table/Fig-1]. All species were predominant in males than females. The most common risk factor among paediatric age group was I/V line (92%) followed by premature birth (73.03%), low birth weight (65.17%) and use of prior antibiotics (60.67%) [Table/Fig-3,4]. In adults, the most common risk factor was central venous line (90.9%), followed by ICU admission (63.64%), prior use of antibiotics (45.4%), patients on ventilator (36.4%), and immune-compromised status (18.18%). Antifungal susceptibility results revealed that C. albicans (85.3%) and C. parapsilosis (85.7%) were found to be most sensitive to fluconazole followed by C. tropicalis (67.4%). All Candida isolates were 100%sensitive to voriconazole. For olive oil, sensitivity of C. albicans was 53.65%, C. tropicalis was 48.83%, C. kruseiwas 55.55% and C. parapsilosis was 57.12%. Sensitivity to Cinnamon oil was 58.83% for C. albicans, 46.51% for C. tropicalis, 44.45% for C. krusei and 57.12% for C. parapsilosis. The resistance to fluconazole as well as olive oil and cinnamon oil was significantly higher (p-value > 0.05) in non albicansCandida spp. as compared to C. albicans [Table/Fig-5,6 and 7].
Major predisposing factors among paediatric patients with Candidemia.
| Predisposingfactors | C. albicans(n= 38) | C. tropicalis(n= 38) | C. krusei(n= 7) | C.parapsilosis(n= 6) | Total(89) |
|---|
| Intravenousline (82) | 36 (43.9%) | 35 (42.7%) | 6(7.3%) | 5(6.1%) | 82(92.13%) |
| Priorantibiotics (54) | 25 (46.3%) | 22(40.7%) | 4(7.4%) | 3(5.56%) | 54(60.67%) |
| Prematurity(65) | 27 (41.5%) | 30(46.15%) | 5(7.7%) | 3(4.6%) | 65(73.03%) |
| Low birthweight (58) | 26 (44.8%) | 25(43.1%) | 5(8.6%) | 2(3.4%) | 58(65.17%) |
Predisposing risk factors among pediatric patients.
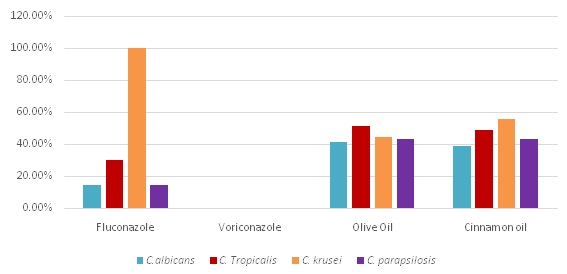
Antifungal resistance pattern against fluconazole, voriconazole, olive oil and cinnamon oil among various Candida species.
| Drugs | C. albicans(n=41) | C. tropicalis(n=43) | C. krusei(n=9) | C. parapsilosis(n=7) |
|---|
| Fluconazole | 6 (14.6%) | 13 (30.3%) | 9 (100%) | 1 (14.3%) |
| Voriconazole | 0 | 0 | 0 | 0 |
| Olive oil | 17 (41.46%) | 22 (51.16%) | 4 (44.44%) | 3 (42.85%) |
| Cinnamon oil | 16 (39.02%) | 21(48.83%) | 5 (55.55%) | 3(42.85%) |
Resistance pattern of various condida isolates against antifungal drugs and oils.
Comparison of antifungal resistance pattern of Candida albicans and non-albicans Candida.
| Antifungal agents | C. albicans | Non albicans Candida | p-value |
|---|
| Fluconazole | 6 (14.63%) | 23 (38.93%) | 0.238 |
| Voriconazole | 0 | 0 | |
| Olive oil | 17 (41.46%) | 29 (49.15%) | 0.083 |
| Cinnamon oil | 16 (39.03%) | 29 (49.15%) | 0.593 |
Discussion
Candidemia has emerged as an alarming problem in healthcare settings since last few decades causing increased morbidity and mortality [10]. In our study, the rate of isolation of Candida spp. from blood stream infections was 7.25%. Our study co-relates with previous studies conducted in India and Thailand, which reported rate of Candidemia as 6.9% and 6.14% respectively [11,12]. In contrast to our study, few studies from Pennsylvania and Saudi Arabia showed isolation rates as low as 3.5% and 2.8% respectively [13,14]. The difference in isolation rates in these studies could be due to the difference in antibiotic policies in different hospitals, as injudicious use of antibiotics leads to suppression of normal flora making the patients prone to develop Candidemia. The various risk factors for acquisition of Candidemia can be ICU settings, extremes of age and previous antibiotic therapy [15]. Our study showed a higher rate of Candidemia (72%) in ICU settings as well as in neonates (36%). Various studies across the globe have also observed a higher incidence of Candidemia in ICUs as compared to non ICU locations [1,15]. Ours is a tertiary care hospital and higher rate of Candidemia in our ICU can be explained by the fact that most patients were already on antibiotics, moreover about 48% of our study population consisted of neonates who generally have a suppressed immune system. Higher rates of Candidemia in neonates have been documented by various studies done earlier also [16,17]. In our study, the most common species was C. tropicalis followed by C. albicans, C. krusei and C. parapsilosis co-inciding with the observations from various other studies, which also depict a shifting trend from albicans to non-albicansCandida spp [1,18,19]. The rate of fluconazole resistance in this study is higher than the previous study done at our center as well as at some other parts of North India [20,21]. There is growing evidence that increasing use of azole agents may be associated with increasing fluconazole resistance as well as emergence of non-albicansCandida spp [22]. The high rate of resistance in the present study may be due to the irrational and liberal use of fluconazole or acquisition of resistance by previously susceptible strains of C. albicans following long term azole exposure. Some non- albicansCandida spp like C. krusei and C. glabrata exhibit resistance to traditional triazole antifungals like fluconazole. Voriconazole can be used effectively for the patients with infections due to C. kruesi and fluconazole resistant voriconazole susceptible C. glabrata, however, these spp may also demonstrate cross resistance to voriconazole resulting in treatment failure [23,24]. Such reports have encouraged looking for the alternative new approaches with low potential for development of resistance. We studied the efficacy of cinnamon oil and olive oil against Candida spp including C. krusei which is intrinsically resistant to fluconazole. Besides having antimicrobial properties, these compounds have other beneficial health effects including antioxidant and anti-inflammatory properties and most importantly free from any systemic side effects [25]. In our study although 50% of the isolates were resistant to both the oils but encouraging finding was that about 55.5% of C. krusei strains were sensitive to olive oil and cinnamon oil. Similar study, has also demonstrated that cinnamon oil and olive oil have marked antimicrobial activity against the fluconazole resistant C. albicans strain (ATCC-10231) [26]. In the present study all the C. krusei strains from Candidemia patients were sensitive to voriconazole but cross resistance may lead to voriconazole resistance resulting in treatment failure [23,24]. Thus these plant oils can find use as anti-Candida agents in future against azole resistant strains.
Limitation
One of the potential limitations of the study is that we did not perform in vivo study. In vitro and in vivo results may not correspond with each other.
Conclusion
We conclude that fluconazole resistant non-albicansCandida has emerged as a major cause of Candidemia especially in neonates and ICU patients. Voriconazole still continues to be a promising drug at our center. Cinnamon oil and olive oil showed marked sensitivity against the fluconazole resistant C. krusei.
[1]. Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, Prasad K, Non-albicans Candida species in blood stream infections in a tertiary care hospital at New Delhi, IndiaIndian J Med Res 2012 136:997-1003. [Google Scholar]
[2]. Sanglard D, Emerging Threats in Antifungal-Resistant Fungal PathogensFrontiers in Medicine 2016 3:11-13. [Google Scholar]
[3]. Upadhyay RK, Dwivedi P, Ahmad S, Screening of antibacterial activity of six plant essential oils against pathogenic bacterial strainsAsian J Med Sci 2010 2:152-58. [Google Scholar]
[4]. Rathi SG, Bhaskar VH, Patel PG, Invitro Anti fungal Screening of Embelia ribes Plant Extract through EUCAST MethodInt J PharmaSci and Research 2010 1:134-38. [Google Scholar]
[5]. Milne LJR, Fungi. In: Collee JG, Fraser AG, Marimon BP, Simmons A, editorsMackie and McCartney: Practical Medical Microbiology 1996 14th edNew YorkChurchill Livingstone:714-17. [Google Scholar]
[6]. Chander J, Fungal culture media. In: Chander J, editorTextbook of Medical Mycology 2009 3rd editionNew DelhiMehta Publishers:508-13. [Google Scholar]
[7]. Reference method for broth dilution testing of yeast approved standard. 2nd ed. Wayne, PA: NCCLS; 2002. National Committee for Clinical Laboratory Standards. M.27-A2 [Google Scholar]
[8]. Fiori ACG, Schwan-Estrada KRF, Stangarlin JR, Vida JB, Scapim CA, Guz MES, Pascholti SF, Antifungal Activity of Leaf Extracts and Essential Oils of some Medicianal Plants against DidymellabryonialJ. Phytopathol 2000 148:483 [Google Scholar]
[9]. Junior A, Zanil C, Biological Screening of Brazilian medicinal plantsBra J Sci 2000 95:367-73. [Google Scholar]
[10]. Giri S, Kindo AJ, A review of Candida species causing blood stream infectionsIndian J Med Microbiol 2012 30:270-78. [Google Scholar]
[11]. Xess I, Jain N, Hasan F, Mandal P, Banerjee U, Epidemiology of Candidemia in a tertiary care centre of North India: 5 year studyInfect 2007 35:256-59. [Google Scholar]
[12]. Tritipwanit K, Chindamporn A, Suankratay C, Epidemiology of Candidemia at King Chulalongkorn Memorial Hospital, ThailandJ Infect Dis Antimicrobial Agents 2005 22:59-69. [Google Scholar]
[13]. Jose A, Rodrigue N, Kusne S, Manez R, Irish W, Linden P, Factors associated with the development of Candidemia and Candidemia related death among liver transplant recipientsAnnals of Surgery 1996 223:70-76. [Google Scholar]
[14]. Osaba AO, Al-Mowallad AW, McAlear DE, Hussein BA, Candidemia and the susceptibility pattern of Candida isolates in bloodSaudi Med J 2003 24:1060-63. [Google Scholar]
[15]. Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J, Epidemiology of Candidemia in Swiss tertiary care hospitals: Secular trends 1991-2000Clin Infect Dis 2004 38:311-20. [Google Scholar]
[16]. Deorukhkar SC, Santosh S, Species distribution and antifungal susceptibility profile of Candida species isolated from bloodstream infectionsJ Evolution Med and Dental Sci 2012 1:241-43. [Google Scholar]
[17]. Amrutkar PP, Rege MD, Chen H, Gentry LO, Garey KW, Comparison of risk factors for Candidemia versus bacteraemia in hospitalized patientsInfection 2006 34:322-27. [Google Scholar]
[18]. Shivaprakasha S, Radhakrishnan K, Karim PMS, Candida spp. other than Candidaalbicans: A major cause of fungemia in a tertiary care centreIJMM 2007 4:405-07. [Google Scholar]
[19]. Baradkar VP, Mathur M, Kumar S, Rathi M, Candida glabrata: Emerging pathogen in neonatal sepsisAnn Tropical Med Public Health 2008 1:5-8. [Google Scholar]
[20]. Goel N, Ranjan KP, Aggarwal R, Chaudhary U, Nanda S, Emergence of Non-albicansCandida in neonatal septicaemia and antifungal susceptibility: Experience from a tertiary care centreJ Laboratory Physician 2009 2:1-10. [Google Scholar]
[21]. Mokaddas EM, Al-Sweih NA, Khan ZU, Species distribution and antifungal susceptibility of Candida bloodstream isolates in Kuwait: a 10-year StudyJ Med Microbiol 2007 56:255-59. [Google Scholar]
[22]. Bagg J, Sweeney MP, Davies AN, Jackson MS, Brailsford S, Voriconazole susceptibility of yeasts isolated from the mouths of patients with advanced cancerJ Med Microbiol 2005 54:959-64. [Google Scholar]
[23]. Magill SS, Shields C, Sears CL, Choti M, Merz WG, Triazole cross resistance among Candida spp: case report, occurrence among bloodstream isolates and implications for antifungal therapyJ Clin Microbiol 2006 44:529-35. [Google Scholar]
[24]. Panackal AA, Gribskov JL, Staab JF, Kirby KA, Rinaldi M, Marr KA, Clinical Significance of Azole Antifungal Drug Cross-Resistance in Candida glabrataJ Clin Microbiol 2006 44:1740-43. [Google Scholar]
[25]. Pattnaik S, Pradhan DK, Jana GK, Evaluation of cinnamon oil, peppermint oil, cardamom oil and orange oil as antimicrobial agentsJ Pharm Res 2010 2:414-16. [Google Scholar]
[26]. Devkatte AN, Zore GB, Karuppayil SM, Potential of plant oils as inhibitor of Candidaalbicans growthFEMS yeast research 2005 5(9):867-73. [Google Scholar]