One of the top ten cancers worldwide is oral carcinoma with rank in India, being first among all cancer cases in males and third among females in many regions [1]. They represent approximately 5% of cancers in men and 2% in women; Squamous Cell Carcinoma (SCC) is the most prevalent histological type. Oral SCC often develops after the age of 50, with a highest peak in the sixth decade of life [2]. The treatment modalities prevalent for Oral SCC include either surgery or radiotherapy along with chemotherapy or various combinations of these depending on the grade of cancer, its pathological findings and presentation. Thus radiotherapy is one of the treatment modality which is used depending on disease presentations and resectability [3]. The estimation of radiosensitivity of individual tumours is essential in choosing the treatment and for planning the optimum radiation schedule for each patient. The in-vivo cytological test and the cell surviving fraction for a 2 Gy dose of radiation are well known means for forecasting radiation response [4]. Forecasting is also possible by assessing the radiation induced nuclear histomorphological changes using a serial cytological evaluation [5]. Response of malignant cells to radiation therapy was assessed by various cytological changes in the nucleus such as nuclear enlargement, micro nucleation, nuclear budding multinucleation (MNU), bi nucleation, karyorrhexis, karyolysis, vacuolization and granulation. Radiation induced changes were first reported by Arneson et al., in the year 1935 [6]. The irradiation effects on mucosal cells of oral cancer patients were identified and gradually the abnormal forms of nucleus observed were named as Pyknosis, NB, MNU [7,8]. The recent studies have also tried to find the molecular origin of these alterations [9]. The escalating increase in Micronucleus (MN), MNU, Karyorrhexis and Karyolysis indices with increasing dose of radiation proves that these parameters can be used as indicators for assessing the response of tumour after radiotherapy. The present study was undertaken to establish the relationship between nuclear changes with radiation dose and to investigate the prospect of utilizing them as an assay to predict tumour response to radiotherapy in oral cancers.
Materials and Methods
This cross-sectional study was conducted from 2006 to 2009 after been cleared by the institutional ethical committee. The cases for the present study were selected from the outpatient department of JIPMER hospital being referred for treatment from surgery and ENT OPD for radiotherapy. Fifty patients (age range of 30-65yrs) with histopathologically confirmed oral squamous cell carcinoma (OSCC) treated by radical radiotherapy alone were included in the present study. The region of radiation was head and neck and each patient received 4, 14, 24 and 60 Gy, respectively, at 2nd, 7th, 12th and 30th day. Any patient treated with other modalities, like surgery or/and chemotherapy, along with radiotherapy or having radiation schedules different from the above mentioned were excluded from the study.
A proforma was prepared in order to record the history and general physical examination in respect of each case. The specimen from the site of lesion (buccal mucosa, alveolus, retro molar area) was collected and slides were prepared following the protocol given by Halder et al., for the diagnosis and confirmation of carcinoma [10]. Care was taken to ensure that scraping was only taken from the tumour site, avoiding the adjacent normal mucosa. The pre-treatment scrape smears were collected from the site of lesion in each patient. Subsequently, after the delivery of various radiotherapy fractions, 3 to 4 smears were prepared from the site of lesion in each patient.
For the collection of specimens: The patients were asked to rinse their mouth scrupulously and following that the material was collected from the oral cavity by scraping the buccal mucosa on the affected site using a wooden spatula. The collected specimen was smeared on clean glass slides. After air drying the slides were placed in freshly prepared fixative in the proportion of 3 parts of methanol and one part of glacial acetic acid for 20 minutes. These fixed slides were stained with May-Grunwald and Giemsa stain.
Procedure of Staining: The slides were air dried and fixed with methanol and stained with Giemsa and May-Grunwald’s stain. The fixed slides were kept in May-Grunwald stained for 5-7 minutes. After washing, the slides were counter-stained with Giemsa stain for 8-10 minutes, followed by washing with distilled water and the stained slides were mounted with cover slip and left undisturbed overnight. The slides were observed for nuclear abnormalities under bright field Nikon microscope under various magnifications. Observations were recorded and tabulated. Photomicrographs showing various nuclear anomalies were taken. From each collected sample 500-1000 cells were evaluated.
Nuclear changes were evaluated and the criteria used for identification of the same were as follows:
Micronucleus (MN): Intra-cytoplasmic, DNA staining bodies having slightly lesser staining intensity, less than one-third the size the main nucleus and in vicinity of nucleus but distinctly separate from it [Table/Fig-1].
Photomicrograph showing formation and separation of micronucleus taken at day – 02 after 4 Gy Radiation.

Nuclear Budding (NB): Bodies similar to micronuclei except for the fact that their separation from the main nucleus was indistinct [Table/Fig-2].
Photomicrograph showing nucleus of the squamous cell showing bulging of nuclear material going in for formation of nuclear bud.

Multinucleation (MNU): More than two nuclei in a single cell with no micronucleus or nuclear budding [Table/Fig-3].
Photomicrograph showing cells showing Multinucleation (arrow 1) and Micronucleus (arrow 2) formation after 14 Gy radiation.

Method of Analysis: Five hundred cells from the prepared smears of each patient were assessed for various radiations induced nuclear changes at 4, 14, 24 and 60 Gy and were compared. Variance was analysed within the group and p-value was calculated. This was analysed by Kruskal-wallis one-way Anova-test.
Results
Out of the 50 cases included in the present study 37(74%) were males and 13(26%) were females (Ratio 3:1). The maximum number of patients were in age group of 51-60 yrs (54%) followed by 20% in 41-50 years, least 10% were in age group of 31-40 years [Table/Fig-4].
Case distribution with age & sex.
| Age Range (yrs) | Males | Females | Total |
|---|
| 31-40 | 04 | 01 | 05 |
| 41-50 | 07 | 03 | 10 |
| 51-60 | 20 | 07 | 27 |
| >=61 | 06 | 02 | 08 |
| Total | 37 | 13 | 50 |
There was an increase in the mean values of MN, MNU and a fall in NB mean value at 4 Gy. At 14 Gy radiations an increase of 22% in MN, almost got doubled in MNU i.e. 62%, and NB also shows a rise of 12% from a fall of 16% at 4 Gy [Table/Fig-5]. A gross fall of 20%, 90% was observed at 60 Gy in MN, MNU whereas NB showed a hike of 26%. At 24 Gy a gross fall from 62% to 18% were observed in MNU. MN showed a further hike of 22% and in NB the value was observed to be more than double [Table/Fig-6].
Showing % rise in nuclear abnormalities.
| Day of Rt | Rt Dose (GY) | MN count / 500 cells | NB count / 500 cells | MNU count /500 cells |
|---|
| Mean ± SD | Degree rise % | Mean ± SD | Degree rise % | Mean ± SD | Degree rise % |
|---|
| 0 | 0 | 49.32±6.251 | -- | 41.24±33.04 | -- | 34.08±3.999 | -- |
| 2 | 4 | 70.52±6.923 | 42 | 33.04±5.357 | -16 | 49.48±4.704 | 30 |
| 7 | 14 | 81.30±5.072 | 22 | 39.64±5.587 | 12 | 80.02±5.587 | 62 |
| 12 | 24 | 92.30±3.824 | 22 | 52.40±4.549 | 26 | 89.26±4.549 | 18 |
| 30 | 60 | 82.52±3.190 | -20 | 65.00±6.286 | 26 | 43.88±6.286 | -90 |
Showing the p-value at various comparison levels with various dosage of Radiotheraphy with pretreatment parameters.
| Days | RT Dose (Gy) | MN | NB | MNU |
|---|
| Mean ± SD | p-value | Mean ± SD | p-value | Mean ± SD | p-value |
|---|
| 0 & 2 | 2 | -21.200±6.842 | <0.001 | 8.200±7.376 | <0.001 | -15.400±6.652 | <0.001 |
| 0 & 7 | 14 | -31.980±6.944 | <0.001 | 1.600±6.943 | 0.110 | -45.940±9.927 | <0.001 |
| 0 & 12 | 24 | -42.980±7.574 | <0.001 | -11.160±6.176 | <0.001 | -55.180±7.024 | <0.001 |
| 0 & 30 | 60 | -33.200±7.884 | <0.001 | -23.760±7.909 | <0.001 | -9.800±5.147 | <0.001 |
A constant and continuous gradual change is seen in all the parameters in [Table/Fig-6] but a rise of 1.6 from 0.8 was seen in NB when we compared pre treatment indices with 14 Gy which is not significant otherwise significant p-value were obtained.
Relative increment % was calculated for all nuclear abnormalities
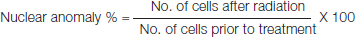
An increase was noted in relative increase index from 145 to 154 for MNU whereas the NB relative index escalated till dose of 24 Gy. At 60 Gy the relative increment index fell down to a level of 2 in both NB and MNU. A marked increase was noted in relative increase index from 143 to 167 in MN [Table/Fig-7].
Relative increment (RI) for nuclear anomalies.
| Days | RT Dose | Mean MN | Mean NB | Mean MNU |
|---|
| 0 | 0 | 49.32 | R.I. | 41.24 | R.I. | 34.08 | R.I. |
| 2 | 4 | 70.52 | 143 | 33.04 | 80 | 49.48 | 145 |
| 7 | 14 | 81.30 | 165 | 39.64 | 96 | 39.64 | 116 |
| 12 | 24 | 92.30 | 187 | 52.40 | 127 | 52.40 | 154 |
| 30 | 60 | 82.52 | 167 | 65.00 | 2 | 65.00 | 2 |
Micronucleus
A significant rise of 42% was observed after administration of 4 Gy radiation to the patient. A serial rise of about 22% was observed at 4 Gy, 14 Gy and 24 Gy. At 60 Gy a fall of 20% in MN was observed. The mean percentage increase of each day (at day 2nd, 7th, 12th and 30th of radiotherapy) when compared with pre-treatment day was statistically significant (p=0.001).
Nuclear Budding
As depicted in [Table/Fig-4], a fall of 16% was observed in NB after administration of 4 Gy and a gradual rise was observed at 14 Gy and 24 Gy as 12% and 26% respectively with no variation at 60 Gy. The paired t-test p-value (p<0.0001) was statistically significant when comparing mean values of NB at various dosage of radiotherapy with pre-treatment values.
Multinucleation
A rise of 30% was observed at administerd dose of 4 Gy, with a peak in the multinucleation at 14 Gy (62%) followed by decrease of 18% and 90%, respectively, at 24 Gy and 60 Gy. The mean percentage increase when compared with pre-treatment day was statistically significant (p=0.001).
Discussion
Radiotherapy is the use of ionizing radiations to treat malignant lesions by causing damage to the DNA and target cells through complicated series of atomic interactions. Most of the nuclear damage is due to the generation of free radicals by the interaction of the radiation with water molecules which in turn interacts with and damages DNA.
In the past, serial cytology has been done in order to find a standard method for the prediction of response to radiotherapy or prognosis of oral cancers by recording the radiation induced cell damage [1]. Response of malignant cells to radiation therapy was assessed by various cytological changes in the nucleus or cytoplasm, named as nuclear enlargement, nuclear budding, micro nucleation, binucleation multinucleation, karyorrhexis and karyolysis.
The present study was undertaken with an aim to evaluate the changes in malignant cells obtained from the scrapings taken from the site of lesion and not from the surrounding tissues. In many previous studies [11,12] the changes were evaluated in benign cells collected from buccal mucosa around the tumour, whereas we have assessed the induction of various nuclear abnormalities in oral carcinoma patients by taking the smears from the site of lesion as we were looking for nuclear changes in the malignant cells.
A total of 50 histopathologically confirmed cases of SCC were included in the present study, 54% of the total cases were in 51-60 years age range and the incidence of oral carcinoma was more in males than in females, 74% and 26%, respectively. Our findings are in accordance with recent literature findings which state that age-standardised incidence rates when stratified by sex were lower in females than males [13], also the male to female ratio is also showing a slow decline, as there is rising incidence in oral cancers in women [14,15]. In our study we found the ratio to be 3:1 which is nearing the ratio cited in literature [16].
Micronucleus: MN are fragments or whole chromosomes, which did not relocate to spindle poles in the process of mitosis and thus remain encapsulated in a separate nucleus [17]. Jaffrey, Silverman, Memon have reported MNU to be the most common radiation induced nuclear change in the cancers of oral cavity but they did not proceed further to explore any kind of correlation of cancer with the radio sensitivity of the tumour if it exists [18,19].
Various studies have observed MN frequencies in buccal mucosal cells of normal, precancerous lesions and those having SCC [20,21]. They concluded that the escalation of MN counts from normal mucosal to precancerous lesions to carcinoma implied a link of this biomarker with neoplastic progression. Ionizing radiation is a treatment modality in many neoplasias but its deleterious side effect includes genetic damage. Some studies have evaluated MN count as a nuclear change and have found the counts to be higher in radiation-sensitive oral tumours than radiation-resistant ones [22]. This has prompted the use of MN counts as a potential predictor of tumour’s radio sensitivity [2].
However, further modified approach is required for the confirmation. Intracytoplasmic bodies representing a fragment, a part or the whole chromosome, resulting genotoxic effect of radiation, identified to be placed near the nucleus, taking up the same stain, around 1/3rd to 1/5th of the size of the main nucleus was found to be in rising titers of 42, 22 and 22 at 4, 14 and 24 Gy, respectively. The maximum increase was observed with 4 Gy dose thereafter increase was 22 at both 14 Gy and 24 Gy. The average number of cells with MN showed a marked and sustained increase, with increase in dose of radiotherapy till 24 Gy but a marked fall in the average number of nuclei was observed with 60 Gy. The rise in the percentage of MN is due to the genotoxic effect of radiotherapy to the tumour cell and -20% fall at 60 Gy could be explained on the basis that cell damage is always accompanied by DNA repair which reduced the damage index of micronucleus in cells. These findings are in agreement with the conclusions of GR Ogden et al., [23].
A similar study was done by L Bindu et al., in which as many as 15 parameters were evaluated, out of which 7 parameters KR, pyknosis, KL, cytolysis, micro nucleation, NB and multi nucleation showed statistically significant results [2]. The evaluation groups in the study by L. Bindu et al., did not include effects of 30th day of Radiotherapy (RT) which we have included in present study to evaluate the degree of maintenance of effect of RT.
Nuclear Budding: In the percentage of NB a marked fall was observed at 4 Gy in all the age groups. Thereafter, a slow and constant rise was observed till 24 Gy which further hiked at 60 Gy (as indicated in [Table/Fig-6]. This could be attributed to the fact that existing nuclear buds got detached from the nucleus and accounted for the MN formation [24]. Probably the NB started off in the newly formed tumour cells which continued and maintained at the same level till 60 Gy. As has been found in various studies concluding that all the nuclear buds showed a dose dependent increase in response to radiation [25,26].
Multinucleation: Multinucleated cell showed a maximum rise from 30% at 4 Gy to 62% at 14 Gy thereafter the rise was only 18% and a gross fall of 90% was observed at 60 Gy [Table/Fig-5]. Two mechanisms responsible for radiation induced MNO have been proposed; firstly radiation induced peroxidation of membrane lipids causing failure of cytoplasmic division leading to formation of a binucleated cell which on further cell division would lead to multinucleation [27]. Secondly, the multipolar mitosis due to damage to pericentriolar matrix has been suggested [28]. In our study, the maximum increase in the multinucleated cells at 14 Gy would probably be the result of these mechanisms but a fall at 60 Gy could be explained on the basis that this massive damage lead to karyorrhexis. Many studies reported that irradiated cells lose their proliferative property which might be because of the fact that hardly any DNA is left after 4 weeks of treatment for cell division [29].
When the paired t-test was applied to the mean values of MN, NB and MNU at various dosage of radiotherapy, significant p-value (p<0.0001) in all the indices was obtained except in the nuclear budding indices between pre treatment and after 14 Gy (p-0.110) as indicated in [Table/Fig-6]. This finding is in agreement with the findings of the previous authors [30].
Relative increment percentage was calculated for all the nuclear parameters taken in the study as per the formula given along with the [Table/Fig-6]. The obtained values shows a marked increase with each dosage of radiotherapy in MN, MNU, till 24 Gy and at 60 Gy a marked fall was observed in MN, where as it remained only two in MNU which was 154 at 24 Gy. From this it is obvious that the proportionate increase or fall in the values of MN, is constant feature so the relative increment percentage of MN, could be used as a biomarker to assess the effectivity of radiotherapy. This consistency in the values of MN, are clearly indicated in the line graphs given in the observations [Table/Fig-8]. In case of NB and MNU the observed data does show a variation in the values but they are not continuous and constant [Table/Fig-9,10].
Micronucleus at 4, 14, 24 and 60 Gy respectively at 2nd, 7th, 12th and 30th day given for different age groups (31-40 yrs, 41-50 yrs and 51-60 years and an average given as blue line).

Nuclear budding at 4, 14, 24 and 60 Gy respectively at 2nd, 7th, 12th and 30th day given for different age groups (31-40 yrs, 41-50 yrs and 51-60 years and an average given as blue line).

Multinucleation at 4, 14, 24 and 60 Gy respectively at 2nd, 7th, 12th and 30th day given for different age groups (31-40 yrs, 41-50 yrs and 51-60 years and an average given as blue line).

Thus the presence of a MN is an accepted test for assessing and monitoring toxicity of chemicals [31] which is very well indicated by its use as a tool marker in diagnosing and identifying the premalignant conditions of oral cavity progressing towards oral carcinoma, caused by continuous use of low doses of alcohol for a longer period or use of tobacco or a combination of both [32,33]. The degree of damage with increasing dose of radiotherapy is probably true for MN induction.
This was reflected in the present study with the MN increasing with radiation dose. The high variance in the micronuclei counts at each dose point suggests the presence of a high degree of intertumoural variation in micronucleus induction [Table/Fig-5]. In present study, on comparing the other abnormalities, significant alteration with each dose of RT were observed and on subjecting the data to t-test it was found to be highly significant. So, use of MN indices can be suggested as a standard parameter for assessing the radiosensitivity and prognosis of the tumour. Even other parameters show significant variations in values. In another study Kumari Rimpu et al., analysed various parameters which showed increased variations in all indices with cumulative doses of radiation [30].
The MN has been found in slides of patients even before the starting of treatment. The presence of MNU in a cell with no known exposure to any genotoxic agent reflects its inherent chromosomal instability, as mitosis is necessary for the expression of MN. The pre-treatment MN count mirrors chromosomal instability and enhanced growth rate of the tumour cells [34]. It has been suggested that gamma rays induced only formation of MN, frequency of which increased linearly with the applied dose, and returns to the initial background levels, a week to twelve days after radiotherapy.
Limitation
The small sampled size and cross-sectional study design are a limiting factor in this study. The study was just to explore the possibility of establishing a relationship between the frequencies of nuclear abnormalities in patients with oral cancer with applied dosage and duration of radiotherapy.
Conclusion
Despite so much progress in cancer treatment, there is little change in the mortality from epithelial or squamous cell carcinoma of oral cavity. The present study was undertaken to explore the possibility of establishing a relationship between the frequencies of nuclear abnormalities in patients with oral cancer with applied dosage and duration of radiotherapy.
The progressive increase in Micronucleus and Multinucleation indices with increasing dose of radiation proves that these parameters can be used as indicators for assessing the response of tumour to radiotherapy. The measurement of relative increment index done in respect to all nuclear abnormalities show a sustained increase with increasing dosage of radiation. The Micronucleus, Multinucleation, indices taken at 4 Gy may be used to select the line of treatment by differentiating the radio-resistant and radiosensitive tumours. These parameters can be used as prognostic indicator in oral carcinoma cases undergoing radiotherapy. The level of response of tumour to radiotherapy as assessed on 7th day can be used for bringing out alteration or modifications in the further treatment.