Oral Squamous Cell Carcinoma (OSCC) continues to impose a serious threat to oral health all over the world. The development of cancer in the oral mucosa occurs in two steps initiated by a potentially malignant disorder that is subsequently followed by oral cancer. Oral leukoplakia a well known potentially malignant disorder has a malignant transformation rate of 3.6 -17.5% [1–3].
The surrounding stroma of the tumour is gaining importance because of its growth and diffusion, with the inflammatory cell infiltrate being actually responsible for cancer progression [4].
Mast cells that are recruited by tumours and which accumulate in the stroma are an important component of cancer-stromal interaction. Many molecules are secreted by mast cells through a discrete and selective pathway of cell secretion known as “piece meal degranulation”. This is a characteristic trait of mast cell activation in chronic inflammatory settings, like cancer for instance and could aggravate the tumour growth. However, mast cells are also found to be helpful in tumour inhibition as the tumour -stroma microenvironment could alter the phenotypic behaviour of mast cells [5,6].
Comprehending mast cells function in cancer progression cannot only improve prognosis but can also develop certain therapeutic methods that target mast cells. Therefore, the present study was undertaken to compare the mast cell count in normal oral mucosa, leukoplakia and OSCC and to evaluate the possible role of mast cells in carcinogenesis.
Materials and Methods
Samples: The study material for the present study comprised of 50 formalin fixed paraffin embedded biopsy specimens retrieved from the Department of Oral and Maxillofacial Pathology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai. The study was approved by the Institutional ethics committee. The archival materials comprised of previously histopathologically diagnosed 20 cases of leukoplakia and 20 cases of well differentiated OSCC. 10 normal gingival samples were used as a control group.
Skin tissue sections formed the positive and the negative control for mast cells and were treated in the same manner as the test groups except that the primary antibody was omitted in the negative control.
Methods: Immunohistochemical study was conducted to assess the mast cell count using mouse monoclonal anti -mast cell tryptase antibody (BioGenex, San Ramon, CA). In brief, 4μm tissue sections were made onto poly- L- Lysine coated slides and deparaffinized in xylene and rehydrated with graded alcohols. Antigen retrieval was done with the help of pressure cooker where the sections were immersed in citrate buffer solution and heated for 15 minutes and allowed to cool at room temperature followed by washing the slides in Tris buffer thrice, each for 15 minutes. Endogenous hydrogen peroxide activity was blocked by treating the sections using peroxide block for 15 minutes in room temperature and background staining was blocked by performing power block for 15 minutes. Incubation of the sections were done using primary mouse monoclonal anti-mast cell tryptase antibody (BioGenex, San Ramon, CA) for 30 minutes and then followed by secondary antibody- super enhancer and lastly Poly HRP ((BioGenex, San Ramon, CA) for 30 minutes each. After the excess being wiped off, the sections were washed with TBS for two changes and then incubated with DAB substrate chromogen (BioGenex, San Ramon, CA) for 5 minutes. Finally the slides were counterstained using Harris haematoxylin and bluing was done in running tap water followed by air drying and mounting with DPX. Evaluation of the stained slides was then carried out under the light microscope.
Evaluation: The anti-mast cell tryptase antibody stained the mast cells reddish brown against a blue background. The number of mast cells in normal mucosa, leukoplakia and OSCC samples in 5 fields at a magnification of x400 at the “hot spots” (areas of increased mast cells) was counted under the light microscope. Mast cell granules that appear reddish brown and one cluster of mast cell granules separable clearly from another adjacent cluster were regarded as a single mast cell. Results were expressed as the average number of mast cells per high power field. All counts were performed by a single investigator to eliminate interobserver variation.
Statistical Analysis
The data was analysed using NCSS Dawson 2007 software. Kruskal Wallis test was used for overall comparison between the groups. A p -value of <0.05 was considered to be significant. Intergroup comparison was done using Mann-Whitney U test. As intergroup comparisons was made between 3 groups, a p-value of <0. 017 was considered to be statistically significant.
Results
The anti-mast cell tryptase antibody showed the presence of reddish brown mast cells in normal oral mucosa [Table/Fig-1], leukoplakia [Table/Fig-2] and OSCC [Table/Fig-3]. The mast cells were identified as round, ovoid, elongated or spindle shaped.
Immunohistochemical demonstration of few intact mast cells in normal oral mucosa using mouse monoclonal anti -mast cell tryptase antibody (x400).
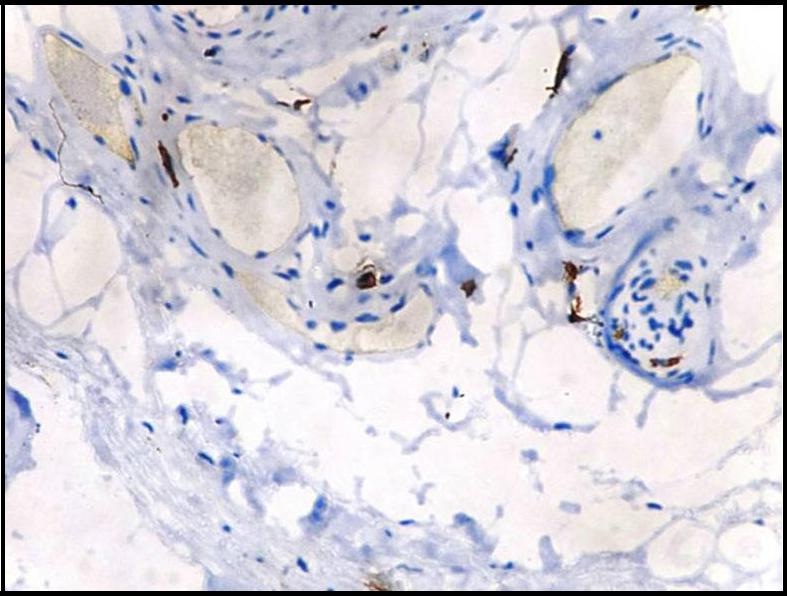
Immunohistochemical demonstration of mast cells in leukoplakia using mouse monoclonal anti -mast cell tryptase antibody (x400).
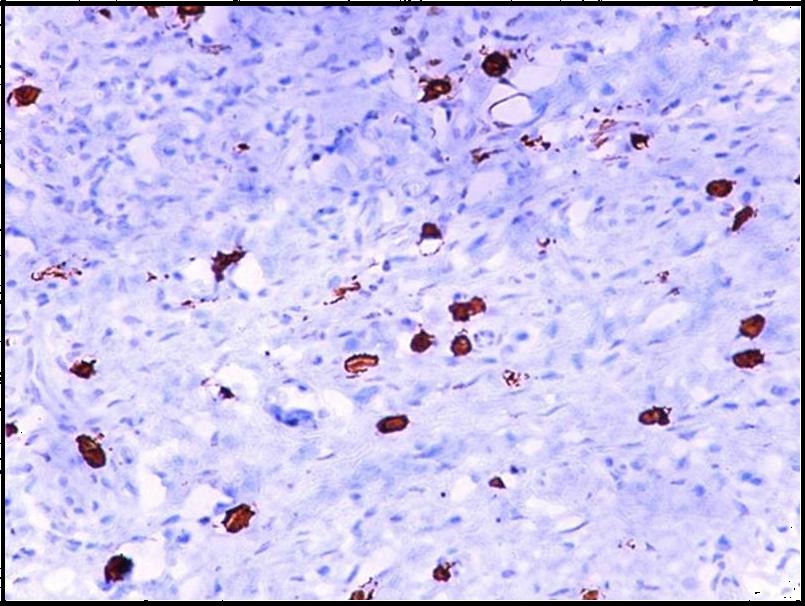
Immunohistochemical demonstration of mast cells in oral squamous cell carcinoma using mouse monoclonal anti - mast cell tryptase antibody (x400).
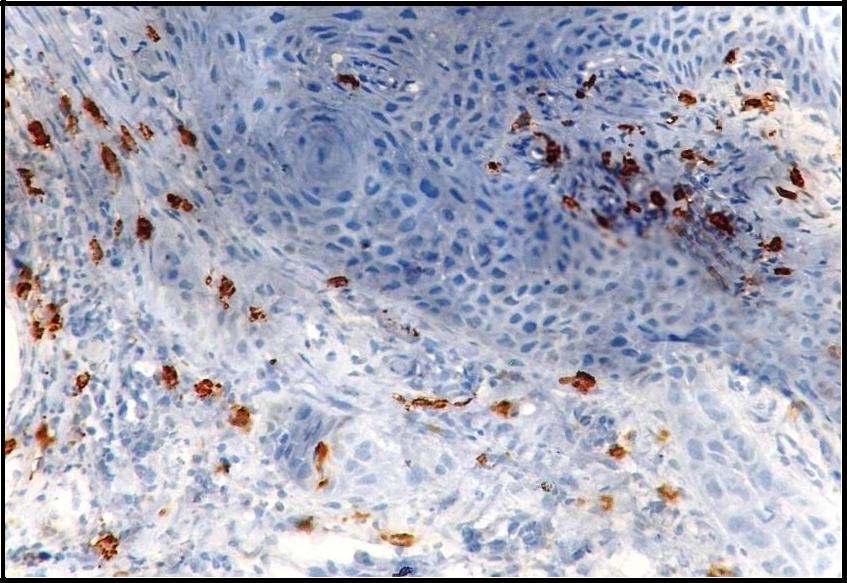
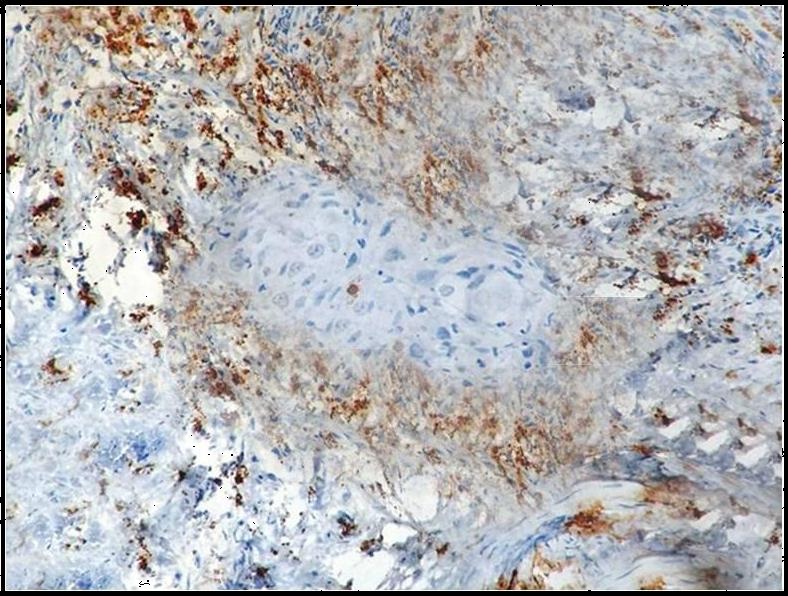
Both intact and degranulated mast cells were observed. Leukoplakia and normal oral mucosa showed the presence of intact mast cells ([Table/Fig-1,4] respectively) compared to OSCC which revealed an increase in degranulated mast cells [Table/Fig-5]. In OSCC the mast cells were seen surrounding the tumour islands [Table/Fig-6].
Immunohistochemical demonstration of intact mast cells in leukoplakia using mouse monoclonal anti -mast cell tryptase antibody (x400).
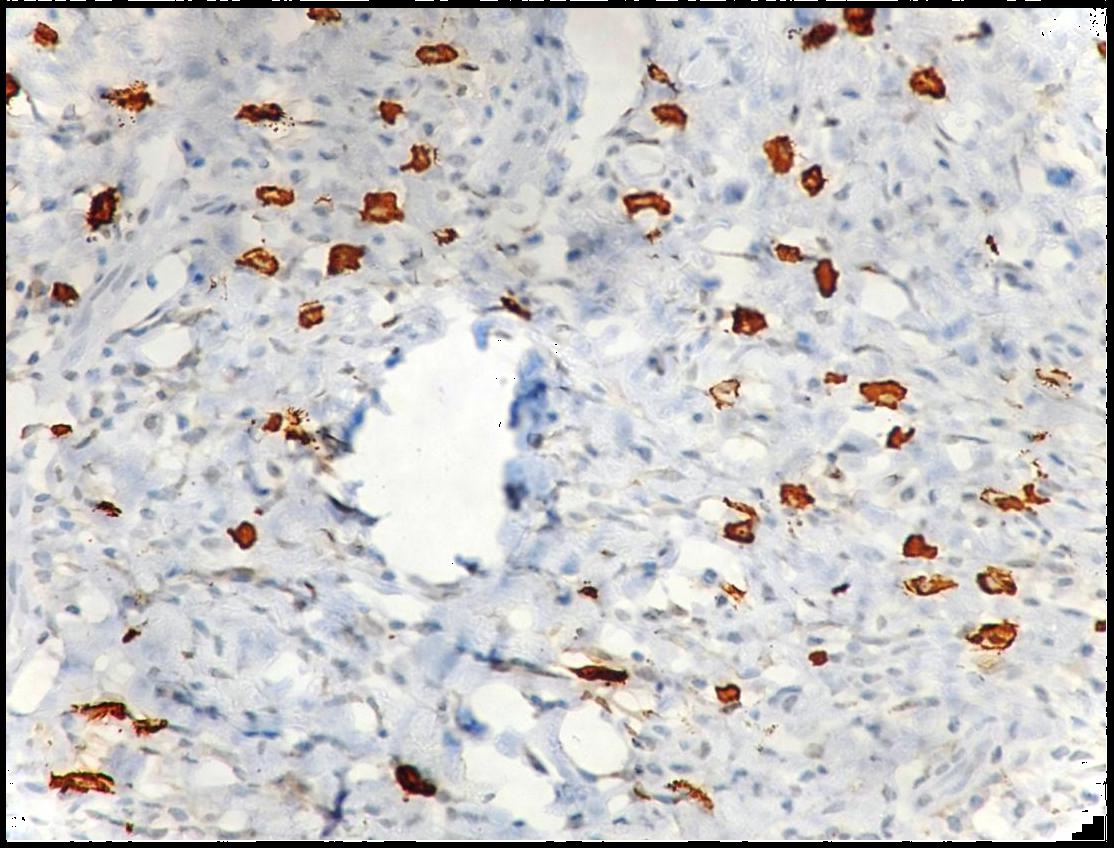
Immunohistochemical demonstration of degranulated mast cells in oral squamous cell carcinoma using mouse monoclonal anti -mast cell tryptase antibody (x400).
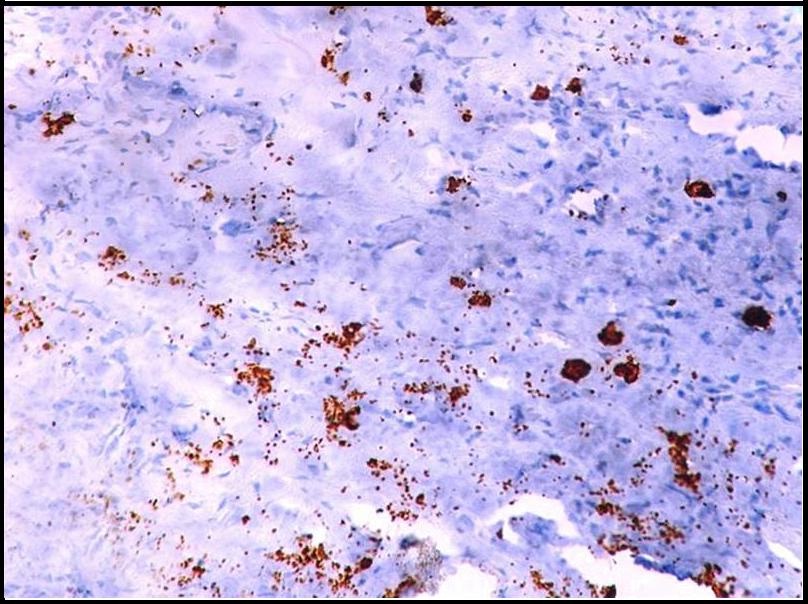
Immunohistochemical demonstration of mast cells surrounding tumour islands in oral squamous cell carcinoma using mouse monoclonal anti -mast cell tryptase antibody (x400).
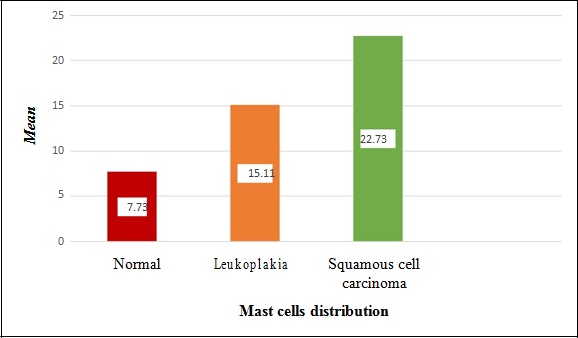
In the present study the number of mast cells were significantly high in OSCC (Mean:22.73) compared to leukoplakia (Mean:15.11) and normal oral mucosal tissues (Mean: 7.73) [Table/Fig-7]. Comparison of mean number of mast cells among three groups were performed using Kruskal Wallis test and it was statistically significant. (p-value:<0.001) [Table/Fig-8]. Intergroup comparisons using Mann-Whitney U test was performed among the three study groups which revealed that there was a significant difference among the three groups in their mast cell count distribution [Table/Fig-9].
Mast cell distribution between study groups.
Comparison of mast cell distribution among study groups using Kruskal Wallis test.
| Groups | N | Mean mast cell count | Standard Deviation | p-value# |
|---|
| Group I | 10 | 7.73 | 2.78 | 0.001 (Significant) |
| Group II | 20 | 15.11 | 7.03 |
| Group III | 20 | 22.73 | 6.45 |
# p-value is significant below the value of 0.05
Inter group comparison of mast cell distribution using Mann - Whitney U test.
| S.No. | Comparison of groups | Median | Mann - Whitney U test | p-value* |
|---|
| 1 | Group II vs Group III | <0.0013 (Significant) |
| Group II | 15.4 | 172 |
| Group III | 22.3 |
| 2 | Group III vs Group I | <0.001 (Significant) |
| Group III | 22.3 | 199 |
| Group I | 6.6 |
| 3 | Group II vs Group I | 0.0008 (Significant) |
| Group II | 15.4 | 311 |
| Group I | 6.6 |
*p-value of <0. 017 was considered as statistically significant
Discussion
Understanding the phenomena behind neoplastic transformation which is expanding and the concept of microenvironment was introduced only recently, since then there is increasing awareness of the interactions between cancer and its microenvironment with multiple cell types in the stromal microenvironment involved in tumourigenesis. The production of chemokines and other chemotactic factors which are further produced by the tumour and the stromal cells are responsible for the number and types of cells composing the inflammatory and immune cell infiltrate in solid tumours [7,8].
Mast cells which are an important source of cancer-stromal interaction exert their tumorigenic effect by their unique ability to secrete certain mediators through limited or “piecemeal degranulation”. They are involved in tumourigenesis through four mechanisms: 1) Angiogenesis; 2) Immunosuppression; 3) Degradation of extracellular matrix; and 4) Release of growth factors [4].
In our study, we used monoclonal anti-mast cell tryptase antibody to evaluate the possible role of mast cells in carcinogenesis and analysed the mast cell count in normal oral mucosa, leukoplakia and OSCC. Special stains such as toluidine blue as well as immunohistochemistry techniques are used to identify mast cell granules but the specificity for immunohistochemical technique has proven to be higher than metachromatic staining in the study done by Oliveira - Neto et al., [9], where mast cells stained by toluidine blue did not show any significant difference between the study groups and in addition tryptase positive mast cells number were higher significantly on comparing with toluidine blue stained mast cells amongst control and leukoplakia groups. Precise staining for human mast cell detection is tryptase immunostaining [10].
In the present study, the highest mean mast cell count was found in OSCC followed by leukoplakia and normal oral mucosa. Similar results were demonstrated in the study done by Michailidou et al., where the mast cell density was found to be highest in OSCC followed by leukoplakia with least number of mast cells in normal oral mucosa [11]. Bhushan Sharma et al., suggested that chemo attractants that are induced by tumour cells or normal connective tissue cells as a response to the tumour can be a reason for increase in mast cells in OSCC [2]. He also stated that an allergic reaction to the different tobacco products that disperse into the connective tissue or cellular injury brought about by the tobacco products can further contribute to the increase in mast cell count in OSCC.
An increased mast cell count in OSCC was also demonstrated by Madhuri Ankle et al., [12] who proposed that mast cell mediators in OSCC play various roles. TNF - α causes increased synthesis of matrix metalloproteinases like collagenase which causes destruction of the basement membrane leading to invasion of epithelial cells into the connective tissue, IL - 1 causes increased T and B cell proliferation thereby leading to increased plasma cell and lymphocytic infiltration and heparin causes tumour angiogenesis resulting in increased vascularity of the stroma in OSCC.
The key role played by mast cells in carcinogenesis is through stimulation of angiogenesis or neovascularization which is fundamental for tumour growth. Mast cells produce several potent pro-angiogenic factors such as histamine, heparin, chymase, bFGF, VEGF, TGF -β, TNF - α and IL- 8. Mast cell tryptase that we used in our study has also been reported as a potent angiogenic factor. Mast cells also recruit other cells to the tumour site such as macrophages, lymphocytes and are involved in the activation of platelets and these non -mast cells also secrete pro -angiogenic factors. Carcinogenesis of squamous cells also leads mast cells to induce neovascularization [2,6,13].
Nooshin Mohtasham et al., in his study demonstrated that mast cell count and the degree of angiogenesis can potentially be used as indicators for the evolution of OSCC from epithelial dysplasia [14].
Although angiogenesis is the primary contribution of mast cells in tumour development, it is not the only one. Mast cell indirectly favour tumour growth through release of growth factors like Colony Stimulating Factor (CSF), Fibroblast Growth Factor (FGF), Nerve Growth Factor (NGF), Transforming Growth Factor (TGF), Platelet Derived Growth Factor (PDGF), and Stem Cell Factor (SCF). They also contribute to tumour growth by promoting immunosuppression through secreting immunosuppressants like histamine, tumour necrosis factor - α, transforming growth factor -β and interleukin -10. Molecules secreted by mast cells are also involved in degradation of extracellular matrix like mast cell proteases such as tryptase and chymase. Tryptase is involved in degradation of connective tissue matrix to providing space for neovascular sprouts. It also acts indirectly by activating latent matrixmetalloproteinases (MMPs) which degrades the extracellular matrix [4–6].
It is also instructive to look at the other side of the coin. Some researchers have also shown anti-tumour activities of mast cells such as cytotoxic functions and release of anti -tumour compounds [2].
Oliveira - Neto et al., found mast cell density to be increased in normal controls compared to OSCC and premalignant lesions in contrast to our results and this can possibly be due to a change in the microenvironment during tumour initiation and progression which resulted in a migration failure of mast cells [9]. Mast cell: tumour ratios was greater than 20:1 and when the cytotoxic effects of mast cells were rendered ineffective the development of tumour intensified and the mast cell - tumour ratios increased to 10:1 to 1:100, these cell mediated cytotoxic effects of mast cells have been reported as stated by Tomita et al., [15]. Therefore the concentration of the mast cell products in the tumour microenvironment is responsible for the effect of mast cells against cancer cells.
Various studies have been carried out to evaluate the role of mast cells in oral cancer. Results of studies similar to present study have been tabulated [Table/Fig-10] where the mast cells were found to be increased in OSCC followed by potentially malignant disorders and normal mucosa. Current study was carried out to compare the mast cell count amongst the study groups and a different attempt was made to correlate their number and type with disease progression, thus assessing their role in carcinogenesis.
Mast cell distribution between the study groups in similar studies.
| Author nameand year | Mast cell count/density± Standard deviation(expressed as cells/mm2) |
|---|
| Normal | Potentially malignantdisorders | OSCC |
|---|
| MohtashamN et al.,2010 [14] | 4. 1±1.7 | 9.3±2.6 | 11.2±2.3(Low grade) | 12.2±3.3(Highgrade) |
| Iamaroon Aet al.,2003 [7] | 41.67±15.38 | 65.83±14.29 | 85.00±27.35 |
| Kinra M etal., 2012 [16] | Mast cellcount | 2.51±0.84 | 5.07±1.71 | 8.65±3.38 |
| Mast celldensity | 12.56±4.18 | 25.35±8.58 | 42.75±17.48 |
| Khare A et al.,2015 [17] | 5.5±5.4 | 12.1±15.4(Nondysplasia) | 12.8± 15.5(Dysplasia) | 13.7±16.5 |
| Veda H et al.,2015 [18] | 7.460±1.223 | 12.215±2.013 | 17.275±4.520 |
Our findings revealed the increased presence of degranulated mast cells in OSCC [Table/Fig-5] with the other study groups showing intact mast cells. This supports the important role of mast cells in carcinogenesis which is their unique ability to release certain mediators selectively through “piecemeal degranulation”. Most important of which is mast cell tryptase that is involved in degradation of extracellular matrix facilitating invasion, with our study further showing the presence of mast cells surrounding the tumour islands in OSCC [Table/Fig-6].
A gradual increase of mast cells from normal oral mucosa to leukoplakia to OSCC was observed. This proves that mast cells might indeed favour malignant transformation probably through mast cell tryptase and can be used as indicators for the evolution of oral OSCC from leukoplakia.
Piece meal degranulation and the role of tryptase have not been discussed much in the past. Thus, intensifying the cytotoxic functions of mast cells and selectively suppressing angiogenesis, tumour promoting molecules, tissue remodelling and prevention of immunosuppression might make mast cells a new target for the adjuvant treatment of tumours. Further studies with larger samples and also considering other potentially malignant disorders for comparison will shed light on the pathophysiologic function of mast cells in carcinogenesis.
Limitation
The, limitation of our present study is that it is not of a large sample size and we have taken only leukoplakia into consideration amongst the potentially malignant disorders.
Conclusion
Thus we observed in our study, that mast cells may play a role in tumour progression and can potentially be used as indicators for disease progression. Together with the study results and literature evidences, mast cells may be considered as a desirable prognostic factor in oral squamous cell carcinoma.
Mast cells thereby represents a target in tumorigenesis, by blocking their pro-tumour mediators and strengthening their cytotoxic functions which constitutes a novel therapeutic approach in oral cancer.
# p-value is significant below the value of 0.05
*p-value of <0. 017 was considered as statistically significant