Meningiomas are the most common neuroepithelial tumours comprising 24-30% of all Central Nervous System (CNS) neoplasms with a five year survival rate ranging from 73-94%. Though meningiomas are generally benign and cured by surgery, some of these tumours may recur along with an inherent propensity to undergo malignant transformation and dedifferentiation thus exhibiting a spectrum of increasing histological grades ranging from Grade I meningiomas, atypical meningiomas (Grade II) to anaplastic meningiomas (Grade III) [1].
Although conventional Haematoxylin and Eosin stain (H&E) is recommended for the study of the well-established histological criteria, and is accepted as the gold standard in diagnosing, grading and assessing the prognosis of meningothelial tumours, these morphological criteria may not be accurate in small biopsy specimens. As many as 7-20% of benign (Grade I) meninigiomas are known to recur [2]. At times, considerable variation in the density of mitotic figures exists in different areas of the tumour. Occasionally, mitotic figures may be difficult to detect and several factors may bias assessment leading to poor interobserver reproducibility [3]. Since the assignment of tumour grade and histological type is the foundation on which therapeutic interventions are based, workers have been searching for newer techniques and ancillary methods which can reliably and easily quantify proliferating cells so that an objective method for assessing tumour biology is available [4].
Ki-67 antigen is a non-histone protein expressed in the proliferative phase of the cell cycle (G1, S, G2, and M phases); it is considered to be the most reliable proliferative marker predicting tumour behavior which can easily be detected in formalin fixed paraffin embedded tissue sections. The MIB-1 monoclonal antibody has been frequently used to stain Ki-67 antigen. The proliferative activity of tumour cells as judged by the Ki-67/MIB-1 labelling index may provide a potential correlate of biologic aggressiveness including prognosis and may help in designing treatment modalities [5,6].
Angiogenesis is a key event in tumourigenesis and has been associated with advanced tumour stage and poor prognosis in various malignancies [7]. Studies have demonstrated an inverse relationship between tumour vascularity and patient survival in various sites including CNS [8,9]. It is believed that tumours recruit new blood vessels from existing vasculature due to angiogenic factors secreted by tumour cells or surrounding stromal cells [10]. Quantification of angiogenesis can be done done by estimating intratumoural Microvessel Density (MVD) after immunohisto-chemical visualization of vessels by specific endothelial markers.
The present study was undertaken to measure the proliferative index bby MIB-1 and correlate it with the grade of meningiomas. It also assessed the expression of CD34 in various grades of meningioma and evaluated their angiogenic potential by calculating MVD.
Materials and Methods
The study was a retrospective as well as prospective analysis of 30 surgically resected cases of intracranial meningothelial tumours, from a tertiary care Institute located in north India from January 2007 to April 2012. There were 10 cases each of grade I meningioma, atypical (grade II) meningioma and anaplastic (grade III) meningioma. Clinical and radiological data were analysed.
Paraffin blocks of all the cases of meningioma were retrieved and histologic features reviewed. The tumour was graded and subtyped according with established WHO criteria [1]. Sections of 4μm thickness were cut serially from representative paraffin embedded blocks and subjected to immunohistochemistry by the labeled streptavidin-biotin complex immunoperoxidase methods with the following antibodies: MIB-1 (mouse monoclonal antibody against Ki-67, clone MMI, Dako), CD34 (mouse monoclonal antibody, Dako). Antigen retrieval was induced by heat-induced epitope retrieval method. Freshly prepared 0.3% H2O2 in methanol for 20 minutes followed by three washes in phosphate buffer saline was utilized to block endogenous peroxidase activity. The sections were incubated in the primary antibody for 60 minutes. A 3,3 diaminobenzidine was used as the chromogenic substrate and counter staining was performed with Haematoxylin. Appropriate positive and negative controls were run.
Immunostaining results were evaluated at high magnification (X400) using an Olympus CX41 microscope. Proliferation index was determined using the MIB-1 antibody. The MIB-1 Labelling Index (L1) was calculated by recording the percentage of positively stained tumour cell nuclei out of 1000 tumour nuclei. Distinct nuclear staining was recorded as positive. Regions with the most immunostaining (known as hot spots) were used in the determination of the labeling index.
Measurement of intratumoural MVD was done to evaluate the angiogenetic potential in meningiomas. This was counted by assessing immunohistochemical expression of CD34 in the most vascular areas (known as hot spots) as described by Weidner [11]. The area containing the maximum number of discrete microvessels was first identified using a scanner objective. Individual microvesselswere counted using a high power objective. Statistical analysis was performed using Mann – Whitney U-test. p-value of < 0.05 was considered statistically significant.
Results
Of the 30 patients, males and females comprised 50% each of the cohort. The age of the patients ranged from 18-81 years (Mean ± SD= 56.6 ± 29.84 years). The male to female ratio in grade I, II and III meningiomas was 1:4, 1.5:1, and 2.3:1 respectively.
The duration of symptoms before seeking treatment ranged from 1 month to 13 months. 73% of patients presented with raised intracranial pressure. Seizures were present in 18.4% of patients. 38% of patients had visual disturbances. The parasagittal and falx was the most common location (23%). The Computerized Tomography (CT) findings in 6 out of the 10 conventional meningiomas showed dense calcification. Four cases of atypical and 5 cases of anaplastic meningioma showed features of malignancy in the form of heterogenous contrast enhancement, indistinct margins and areas of intratumoural calcification.
Grading in all cases was done as per WHO 2007 classification of meningiomas. Out of the 10 grade I cases, the following histological subtypes were seen: 4 were meningothelial [Table/Fig-1a], 2 each were fibroblastic and transitional whereas 1 case each showed an angiomatous and psammomatous histology respectively.
(a) Meningothelial (Grade I meningioma) H and E stain X 400 showing synctial and whorl pattern of meningothelial cells having round nuclei and fine chromatin. (b) Endothelial cells and blood vessels stain positive (CD34 X 400). (c) Proliferating tumour cells showing nuclear positivity (MIB-1 X 400).
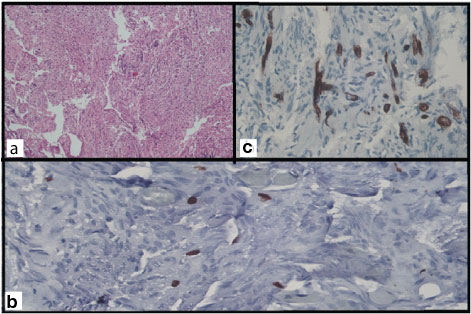
MIB-1 LI in grade I tumours showed scattered, focal positivity [Table/Fig-1b]. In grade II tumours it was uniformly positive [Table/Fig-2a] but in grade III tumours heterogenous staining in the form of densely immunolabelled nuclei and sparsely stained nuclei were distributed haphazardly. Negative control slides showed complete absence of MIB-1 staining.
(a) Atypical (grade II) meningioma, (H&E stain, X400). Increased cellularity diffuse sheeting and areas of necrosis. (b) CD34 X400. (c) MIB-1 X400.
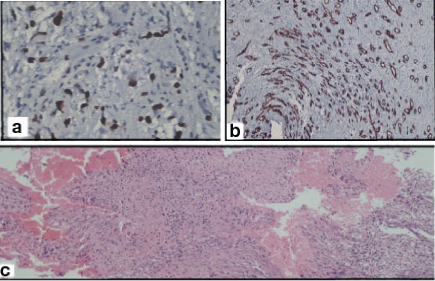
MIB-1 LI ranged from 0% to 42% across the various grades. The mean ± SD MIB-1 LI in grade I tumours was 1.14 ± 0.84 while that in grade II and grade III tumours was 8.94±2.73 and 35.62 ± 4.44 respectively [Table/Fig-3]. The difference in MIB-1 across various grades was found to be statistically significant (p < 0.01) [Table/Fig-4].
Mean ± SD (Ranges) of MIB-1 LI and MVD in various grades of meningioma.
| Grades | GradeI | Grade II | Grade III |
|---|
| Mean +SD(Range) | Mean+SD(Range) | Mean+SD(Range) |
|---|
| MIB1 LI | 1.14±0.84(0-2.5) | 8.94±2.73(4.5-15.5) | 35.62±4.44(28.25-42) |
| MVD(CD34) Vessels/mm2 | 49.67±22.35(26.64-77.68) | 41.37±7.45(30.84-52.44) | 47.86±10.77(28.68-64.86) |
Multivariate analysis of MIB-1 LI between various grades of meningioma.
| Grade I and Grade II | p < 0.01 |
| Grade II and grade III | p < 0.01 |
| Grade I and grade III | p < 0.01 |
CD34 staining was seen to be uniform in grade I tumours whereas both grade II and III tumours showed both densely immunolabelled and sparsely stained endothelial cells distributed haphazardly [Table/Fig-1c,2b,5a].
(a) Anaplastic (grade III) meningioma, H and E stain, X400. Cellular tumour with malignant cytology resembling that of carcinoma and sarcoma. (b) CD34 X400.
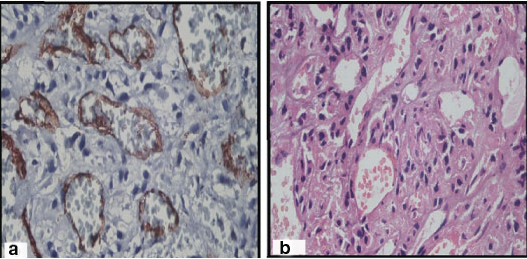
MVD as assessed by CD34 immunostaining ranged from 25.64% to 77.68%. The mean ± SD MVD in grade I tumours was 49.67 ± 22.35 whereas in grade II and III tumours was 41.37±7.45 and 47.86 ± 10.77 [Table/Fig-3] respectively and was not statistically significant.
Discussion
Meningiomas are the tumours arising from meningothelial cells, mostly attached to the inner surface of the dura mater. These tumours are graded into three grades depending upon certain histological features and the presence of increased mitotic activity. Grade I meningiomas are associated with a low risk of recurrence and do not show an aggressive behaviour. The criteria of atypical meningioma (grade II) are increased mitotic figures (more than 4/10HPF), three or more of the following histological features: increased cellularity, sheet like growth, small cells with high N:C ratio and foci of necrosis [Table/Fig-2c]. Chordoid meningioma and clear cell meningioma are also included in this grade. Anaplastic meningioma (grade III) shows obvious features of malignancy with more than 20 mitotic figures per 10 HPF. At times, the appearance of these tumours may resemble a carcinoma or sarcoma [Table/Fig-5b]. Papillary meningioma and Rhabdoid meningioma have a grave prognosis and hence, these are included in grade III [1].
The present study included 30 cases of meningothelial tumours distributed equally across the various grades namely classical (grade I), atypical (grade II) and anaplastic (grade III). Ki-67 and MVD (CD34) in all cases were determined and evaluated against other parameters.
The patients’ ages ranged from 18 to 81 years and overall M:F ratio was 1:1. Grade I tumours predominantly occurred in females. This pattern of age and sex predilection has been observed by other investigators [1,12]. They have described similar skews for female patients in lower grade meningiomas along with an overall predilection for female patients. In our study, no overall female predilection was seen which could be due to the small sample size. CT scan, though useful in demonstrating trends indicative of atypia was not completely reliable in differentiating benign from atypical meningioma in our study. This view has also been echoed by other workers [13].
Psammoma bodies were seen in all except angiomatous histopathological patterns of Grade I meningioma. Comparison of mean MIB-1 LI values showed statistically significant differences (p <0.01) between the three grades of meningothelial tumours and showed positive correlation with tumour grades in grade I,II and III meningiomas [Table/Fig-4]. The use of MIB-1 LI as a prognostic indicator in meningiomas has been the subject of many studies. Most but not all studies have found significant differences between the MIB-1 labelling indices of benign, atypical and anaplastic meningiomas [1,14–18] [Table/Fig-6]. This discrepancy could be explained by the heterogenous nature of these tumours. In this study also, it was observed that MIB-1 staining was not uniform in all sections of grade III tumours.
Comparison of various studies of MIB-1 LI in meningiomas.
| Study | n | Grade I | Grade II | Grade III |
|---|
| Maier et al., [14] | 48 | 0.08±0.05 | 4.8±0.9 | 9.0±4.1 |
| Takei et al., [15] | 68 | 5.7 ±3.73 | 10.01±4.10 | 15.66±12.64 |
| Shayanfar et al., [16] | 78 | 2.98±2.27 | 9.30±5.79 | 34±5.47 |
| UzumNataoglu GA [17] | 246 | 2.61±5.68 | 8.61±11.77 | 11.79±14.06 |
| Present study | 30 | 1.14+/-0.84 | 8.94±2.73 | 35.62±4.44 |
Ho and other workers [19] in a study undertaken in 83 patients of meningioma over 10 years concluded that histopathological findings along with MIB-1 LI were useful parameters that could predict clinical outcomes in these patients. Fifty two tumours with an LI of <10 did not recur within 10 years. Of the 31 tumours with an LI >10, 97% showed a recurrence. Another study demonstrated the MIB-1 LI of recurrent meningiomas as 7.1% whereas the MIB-1 LI of non-recurrent tumours as 3.8% [20].
Studies have also elucidated the problem of overlapping MIB-1 LI values between groups which warrants caution in interpretation of MIB-1 LI in a given tumour. Other proliferative cells like lymphocytes can also show positivity for MIB1 LI, and a meticulous comparison with H and E stain would help to eliminate this error [20,21].
The quantification of MVD i.e., the number of vessels per square mm can help in the evaluation of neoangiogenesis in meningiomas. There is a paucity of studies comparing CD34 immunoreactivity in different grades of meningioma and conflicting results have been obtained regarding its prognostic role in meningiomas when quantified by staining by pan endothelial markers like CD34 [22–24], factor VIII [25], or CD31 [26]. This could be due to the differences in sensitivity, cut off values and the microvessel counting techniques employed. The present study used CD34 to assess MVD which ranged from 25.64% to 77.68%. We did not find any statistical significant difference in MVD across various grades of meningioma (p>0.05). The CD34 yield was strongest in angiomatous meningioma, due to its blood supply. These findings are consistent with the data published by Lamzsuz and other workers who also did not find any correlation between MVD immunoreactivity and WHO grade in meningiomas [27].
Baressi and co workers [28] have proposed that CD105 (endoglin) could be a more specific marker for neoangiogenesis in comparison to pan endothelial markers since CD105 was predominantly expressed by cycling endothelial cells indicating active angiogenesis as seen in tumours, inflamed or regenerating tissues. In a study comprising of 54 cases of both grade I and II meningiomas, the authors found that high CD105 values were significantly correlated with higher histological grades and corresponding higher ki-67 values. The study also compared MVD using CD34 immunoexpression on consecutive parallel sections. It was observed that CD 34 antibody stained blood vessels in both normal leptomeninges and tumour in contrast to CD105 which was positive only in the tumour. Furthermore, CD105 MVD but not CD 34 MVD showed an inverse significant correlation with overall survival and recurrence free survival. Some authors have also reported a significant correlation of MVD with VEGF m RNA expression in meningiomas [26].
Limitation
The present study was limited by its small sample size of 30 cases and lack of patient follow up. A larger sample size along with a long term follow up to check the prognostic significance of MIB1 labelling index and MVD density by CD34 needs to be assessed.
Conclusion
MIB-1 LI is an important complementary tool in neuropathology to accurately grade meningothelial tumours and help to plan treatment modalities especially in small stereotactic biopsies. It would also be of help in case of a discrepancy between grading of these tumours based on morphologic features and neuroimaging. Though CD 34 MVD did not correlate with the grade of the tumour or MIB LI, possibly the use of more specific endothelial markers along with CD34 MVD could help in prognosis and predicting tumour recurrence and also aid in the design of anti angiogenic factors for use in therapeutics.