Comparison of the Effect of Clomiphene- Estradiol Valerate vs Letrozole on Endometrial Thickness, Abortion and Pregnancy Rate in Infertile Women with Polycystic Ovarian Syndrome
Fariba Seyedoshohadaei1, Laleh Tangestani2, Farnaz Zandvakili3, Naser Rashadmanesh4
1 Associate Professor, Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2 Resident, Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3 Assistant Professor, Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
4 Lecturer, Research Center for Gastroenterology and Hepatology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Laleh Tangestani, Keshavarz St, Besat Hospital, Sanandaj, Iran.
E-mail: Laleh.tangestani@yahoo.com
Introduction
Clomiphene citrate is the first-line therapy for ovulation induction in Polycystic Ovarian Syndrome (PCOS). This drug binds and blocks estrogen receptors and thought to have an anti estrogenic effect on endometrium volume, thus may have adverse effect on fertility.
Aim
This study aimed to compare the effect of Clomiphene citrate plus Estradiol Valerate with Letrozole on endometrial thickness, abortion and pregnancy rate in infertile women with PCOS undergoing ovulation induction.
Materials and Methods
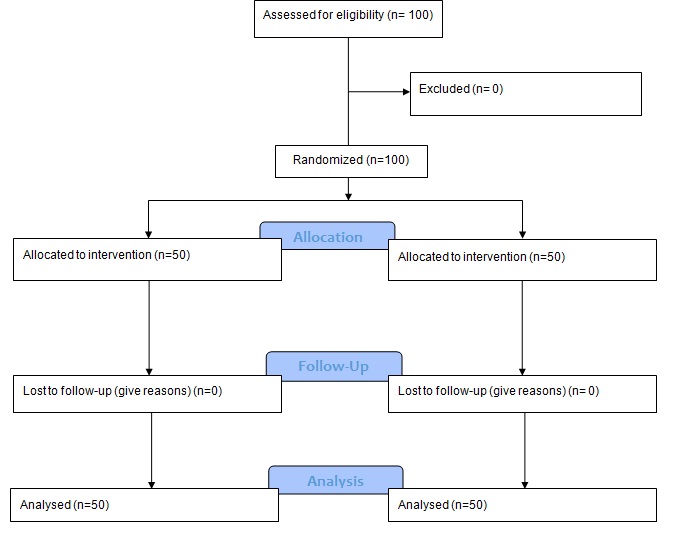
This was a randomized double blind clinical trial study on 100 women with PCOS, with an endometrial thickness less than 7mm in spite of follicles greater than 18mm after administration of Clomiphene citrate 100mg/d from 3th to 7th day of menstruation. They were randomly divided in two groups. Group A received 100mg Clomiphene citrate from day 3 to day 7 of menstruation and 4 mg Estradiol Valerate after the 8th day of menstruation until 14th day. Group B treated by 5mg Letrozole from day 3 to 7 of menstruation with placebo from 8th to 14th day of menstruation. In both groups endometrial thickness was measured by transvaginal sonography in the 14th day of menstruation. Data were analysed using SPSS Ver.18.0.
Results
The mean age was 30.34 years in group A and 29.62 years in group B (p=0.381). The number of infertility years in group A was 3.73 years and in group B was 3.85 years. There was no significant relationship statistically between the two groups in terms of mean age and infertility years (p=0.99). Endometrial thickness in group A was 7.26mm and in group B was 8.17 mm. Pregnancy rates in group A and group B was 32% and 16% respectively. There was significant relationship statistically between the two groups in terms of endometrial thickness and pregnancy rates (p=0.021 and p=0.05). There was no abortion in group A and 5 cases had abortion in group B, there was a significant relationship between the two groups statistically (p=0.028).
Conclusion
Letrozole increased endometrial thickness and pregnancy rate in infertile women, therefore its administration is recommended.
Drug therapy, Female, Infertility
Introduction
Polycystic Ovarian Syndrome (PCOS) is the most common endocrine disorder in infertile women. Infertility affects 40% of women with PCOS [1]. Understanding the main causes of infertility and selecting an appropriate treatment plan is a diagnostic and therapeutic priority [2,3].
Clomiphene citrate is the first line of treatment for ovulation induction in PCOS. The structure of Clomiphene is similar to estrogenic compounds and by blocking estrogen receptors reduces estrogen effect [4]. Previous studies have shown the success rate of 50%-75% of ovulation for this treatment method, but the numbers of pregnancies were less than expected, between 25-43% [5,6].
Undoubtedly the endometrial thickness is one of the most important factors in infertility treatment. The pregnancy rate can be very low, especially if the endometrial thickness is less than 6–8 mm [7]. The reduction in endometrial thickness among treatment modalities containing Clomiphene citrate is widely shown in the literature [8–10]. The use of drugs to improve endometrial thickness is of interest to researchers [11,12]. Endometrial thickness facilitates the embryo replacement, therefore it reduces the risk of spontaneous abortion and also increase pregnancy rate [13]. Physicians have tried to adjust Clomiphene effects using ethinyl estradiol [14]. Appropriate changes in both endometrial thickness and uterine volume growth during the follicular phase in Clomiphene citrate and ethinyl estradiol treatment cycles were observed by Yagel et al., [15]. Khanna showed that adding ethinyl estradiol to treatment protocols that include Clomiphene citrate produce a favourable endometrial response in infertile women with PCOS [16]. In a study by Khadem et al., the use of Clomiphene with ethinyl estradiol had a significant effect on endometrial thickness than those who had used Clomiphene alone, but had not been effective on the pregnancy rate and abortion [17]. However in a study by Sohrabvand et al., favourable changes were not observed from treatment with ethinyl estradiol [18]. Therefore, another drug called Letrozole (an Aromatas inhibitor) which is effective for the treatment of patients resistant to Clomiphene has been taken into consideration [19]. Letrozole induces agonistic effects of estrogen on endometrium rather than antagonistic effect. It induces ovulation by inhibiting the conversion of androgens to estrogen that creates an estrogen-deficient environment [20]. Since this drug unlike Clomiphene does not block estrogen receptors and normal central feedback mechanisms remain intact, therefore, growing dominant follicle increases estrogen levels and by negative feedback decreases FSH, monofollicular growth occurs later [19,20]. The half-life of Letrozole is 45 hours which is much shorter than Clomiphene. This feature of Letrozol provides the better situation for ovulation compared to Clomiphene. Letrozole rarely produce more than one follicle; therefore the risk of multiple pregnancy and Ovarian Hyperstimulation Syndrome is reduced [20,21].
Previous studies showed significant effect of Letrozole on endometrial thickness compared to Clomiphene [22–24]; although there are studies that have not confirmed the difference between these two drugs [25,26]. This study aimed to compare the effect of Clomiphene citrate plus Estradiol Valerate with Letrozole on endometrial thickness, abortion and pregnancy rate in infertile women with polycystic ovarian syndrome.
Materials and Methods
This double blind clinical trial study was conducted on 100 PCOS infertile women who have not responded to initial treatment, referring to the Infertility Center of Sanandaj Besat Hospital from June 2014 to December 2015. They were administered with Clomiphene citrate 100mg/d from 3th to 7th day of menstruation, in spite of follicles greater than 18mm after administration of Clomiphene citrate their endometrial thickness was less than 7mm as diagnosed by transvaginal sonography. The frequency of transvaginal sonography was 9MHz.
The sample size was calculated based on previous studies [3–5]. Considering the mean of endometrial thickness, 5% type I error and 20% type II error, 45 patients were required in each group. To compensate for possible loss and increase the power of the study, 50 patients were studied in each group.
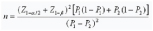
PCOS was confirmed by Rotterdam criteria (menstrual disturbances: oligomenorrhea or amenorrhea, clinical or biochemical hyperandrogenism and sonographic findings of polycystic ovaries). Patients with two of the three PCOS criteria were included in the study. Patients with hyperprolactinemia, thyroid problems and anatomical problem in uterus cavity and fallopian tubes confirmed by Hysterosalpangiography, Sonohysterography or Laparoscopy were excluded from the study. Written consent was taken before the intervention.
Women were block randomized and divided in two groups [Table/Fig-1]. Group A received 100mg Clomiphene citrate (Iran Hormone Pharmaceutical Company) from day 3 to day 7 of menstruation and 4mg Estradiol Valerate (Aburaihan Pharmacy Company) after the 8th day of menstruation until 14th day. Group B treated by 5mg Letrozole (Iran Hormone Pharmaceutical Company) from day 3 to 7 of menstruation with placebo from 8th to14th day of menstruation. In both groups endometrial thickness was measured by transvaginal sonography in the 14th day of menstruation by the same clinician then timed spontaneous coitus was recommended. The patients were followed for pregnancy rate and outcome of pregnancy. To blind the study, transvaginal sonography was performed by a fellow of infertility, the medication was prescribed by a gynaecologist and the patients in group B received placebo.
Flow diagram of the progress through the phases of a two-group parallel randomized trial.
This study was approved by the Ethics Committee of Kurdistan University of Medical Sciences and has been registered in the Iranian Registry of Clinical Trials with registration number IRCT2015052612789N11.
Statistical Analysis
Data were recorded in a data sheet that was prepared for this purpose. Data were analysed using SPSS Ver.18.0. For normally distributed variable (endometrial thickness after treatment), the independent t-test for quantitative variables with non-normal distribution (age, years of infertility and endometrial thickness before treatment) "Mann-Whitney U" test and for other variables (two nominal qualitative variables) "Chi-square" was used. Kolmogorov-Smirnov test was used to determine the distribution of quantitative variables. The significance level of p≤ 05 was determined.
Results
The mean age in group A and group B were 30.34 and 29.62 years (p=0.381). Duration of infertility in group A and group B were 3.37 and 3.85 years (p=0.99). In terms of age and duration of infertility there was no significant difference between two groups statistically [Table/Fig-2].
Comparison of age and duration of infertility in both groups.
| Groups | Clomiphene- EstradiolValerate (A) (n=50) | Letrozole (B)(n=50) | p-value |
|---|
| Age (Years) | 30.34±3.09 | 29.62±5.07 | 0.381 |
| Duration of Infertility (Years) | 3.37±2.77 | 3.85±2.35 | 0.99 |
In group B 36(72%) patients and in group A 30(60%) patients had primary infertility. In general 66% of the study population had primary infertility, but there was no significant difference between two groups in terms of infertility type (primary or secondary) (p=0.205) [Table/Fig-3].
Comparison of the two groups in terms of primary and secondary infertility.
| Type of Infertility | PrimaryNo.(%) | SecondaryNo.(%) | p-value |
|---|
| Groups |
|---|
| Clomiphene- Estradiol Valerate (A) (n=50) | 30 (60.0) | 20 (40.0) | 0.205 |
| Letrozole (B) (n=50) | 36 (72.0) | 14 (28.0) |
| Total | 66 (66.0) | 34 (34.0) |
The mean of endometrial thickness in group A and B before the intervention were 5.34 and 5.68mm. There was no significant difference between two groups statistically (p=0.174). After intervention the mean of endometrial thickness was 7.26 in group A and 8.17mm in group B respectively. There was significant difference between two groups (p=0.021) [Table/Fig-4].
Comparison of endometrial thickness in the two groups before and after the intervention.
| Groups | Endometrial Thickness Mean |
|---|
| Before Intervention | After Intervention |
| Clomiphene- EstradiolValerate (A) (n=50) | 5.34±1.67 | 7.26±1.76 |
| Letrozole (B) (n=50) | 5.68±1.98 | 8.17 ±2.08 |
| P | 0.174 | 0.021 |
The total number of pregnancies was 24 cases (24%). The number of pregnancies in group B (32%) was higher than group A (16%) and there was a significant differences between two groups in terms of pregnancy rate (p=.05). There was no abortion in group A and 5 cases had abortion in group B, there was a significant relationship between two groups (p=.028). Total numbers of live births were higher in the Letrozole group [Table/Fig-5].
Comparison of pregnancy rate, abortion and Live birth in the two groups.
| Groups | Clomiphene- EstradiolValerate (A) (n=50) | Letrozole (B) (n=50) | p-value |
|---|
| No (%) | No (%) |
|---|
| Pregnancy | 8(16.0) | 16 (32.0) | 0.05 |
| Abortion | 0(0.0) | 5 (100) | 0.028 |
| Live Birth | 8 (16.0) | 11(22.0) | 0.135 |
Discussion
The present study showed that endometrial thickness was increased after administration of Clomiphene plus Estradiol Valerate and Letrozole, but there was significantly different in the two groups (p<.05). In Letrozole group endometrial thickness was higher than Clomiphene plus Estradiol Valerate. Previous studies have compared the effects of Letrozole and Clomiphene citrate on ovulation induction. Hendawy et al., showed that Letrozole had a better effect on endometrial thickness and pregnancy rate than Clomiphene citrate [27]. Roy et al., compared the efficacy of Letrozole and Clomiphene citrate in PCOS patients with infertility; they concluded that Letrozole had a better endometrial response and pregnancy rate compared with Clomiphene citrate [28]. In a study by Xi et al., use of Letrozole and Clomiphene citrate combined with gonadotropins in Clomiphene-resistant infertile women with PCOS was evaluated. The first group received the Letrozole + HMG, the second group received Clomiphene citrate + HMG, and the third group received HMG only. The rate of monofollicular development was 80.2% in the Letrozole + HMG group, 65.3% in the Clomiphene citrate + HMG group, and 54.7% in the HMG-only group. The difference between these three groups was significant statistically. Endometrial thickness in the group receiving Letrozole was higher than other two groups [29]. In a study by Sharief and Nafee which was conducted on 75 Iraqi women the findings showed that the number of mature follicles was significantly lower, but the endometrial thickness and ovulation were significantly higher in Letrazole group than in Clomiphene citrate group (p<0.05 each) [30]. Mitwally and Casper found that Letrozole has associated with greater endometrial thickness [31]. In all these studies endometrial thickness was significantly higher in the Letrozole group (p<0.01). The result of our study was in consistent with findings of Kamath et al., Hendawy et al., Roy et al., Xi et al., Sharief and Nafee and Mitwally and Casper [22,27–31].
There are also studies that have not shown significant difference between the two treatments in terms of the endometrial thickness after the intervention [32,33]. The results of a study by Ghomian et al., showed no differences between Letrozole and Clomiphene citrate in terms of the endometrial thickness, the number of mature follicles, and length of follicular phase [33].
In our study the endometrial thickness in Clomiphene plus Estradiol Valerate group was also improved after intervention (5.34mm vs. 7.26mm). In a study by Satirapod et al., the effects of Estradiol Valerate on the thickness of Clomiphene citrate -stimulated endometrium was examined. They concluded that the administration of Estradiol Valerate following the Clomiphene citrate treatment can prevent the endometrial thinning [13].
The results showed that the pregnancy rate in the Letrozole group was almost twice as Clomiphene plus Estradiol Valerate group and the difference was significant. This finding is also consistent with previous studies. Seyedoshohadaei et al., evaluated the effectiveness of Clomiphene citrate, Tamoxifen and Letrozole in ovulation induction in infertile women. The pregnancy rate in Letrozole group was higher than Clomiphene citrate group, but the differences were not significant [19]. In a study by Hendawy et al., pregnancy rate in Letrozole group was higher than Clomiphene citrate group [27]. Kar in a study showed that Letrozole has excellent pregnancy rates compared to Clomiphene citrate [5].
Some studies found that there was no significant difference in pregnancy rate between the two groups [28,34]. Nahid and Sirous compared the effects of Letrozole and Clomiphene citrate for ovulation induction in women with PCOS, they showed that pregnancy rate in both groups was almost similar [35].
The results of present study showed that from 16 pregnancies in the Letrozole group 5 were aborted. In Clomiphene group 8 pregnancies were observed but no case aborted. There was a statistically significant, but fewer pregnancies occurred in Clomiphene group and abortion did not happen. It seems Estradiol Valerate increased endometrial thickness in Clomiphene group but did not affect abortion and pregnancy rate.
In a study by Seyedoshohadaei et al., abortion rate in Clomiphene group was 10 (20%) while it was 4 (8%) in Letrozole group (p=0.05) [19]. Akbari et al., also showed fewer abortion rates in Letrozole group compared with Clomiphene [35].
He and Jiang in a meta-analysis study compared the clinical efficacy and safety of Letrozole with Clomiphene citrate for ovulation induction in women with PCOS; they concluded that Letrozole is as effective as Clomiphene citrate for ovulation induction in patients with PCOS. There were no significant differences in pregnancy, abortion and multiple pregnancy rates between the two groups [36].
Conclusion
Letrozole increased endometrial thickness and pregnancy rate than Estradiol Valerate and Clomiphene citrate in infertile PCOS women who had improper endometrial thickness with Clomiphene citrate, therefore its administration is recommended for above mentioned patients.
[1]. Teede H, Deeks A, Moran L, Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespanBMC Med 2010 8:41 [Google Scholar]
[2]. Speroff L, Fritz M, Clinical gynaecologic endocrinology and infertility 2005 PhiladelphiaLippincott Williams & Wilkins [Google Scholar]
[3]. Reynolds K, Khoury J, Sosnowski J, Thie J, Hofmann G, Comparison of the effect of tamoxifen on endometrial thickness in women with thin endometrium (<7mm) undergoing ovulation induction with Clomiphene citrateFertility and Sterility 2010 93(6):2091-93. [Google Scholar]
[4]. Dehbashi S, Parsanezhad ME, Alborzi S, Zarei A, Effect of Clomiphene citrate on endometrium thickness and echogenic patternsInt J Gynaecol Obstet 2003 80:49-53. [Google Scholar]
[5]. Kar S, Clomiphene citrate or Letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trialJ Hum Reprod Sci 2012 5(3):262 [Google Scholar]
[6]. EL-Gharib M, Mahfouz AE, Farahat M, Comparison of letrozole versus tamoxifen effects in clomiphen citrate resistant women with polycystic ovarian syndromeJ Reprod Infertil 2015 16(1):30-35. [Google Scholar]
[7]. Cetinkaya K, Kadanali S, The effect of administering vaginal estrogen to clomiphene citrate stimulated cycles on endometrial thickness and pregnancy rates in unexplained infertilityJournal of the Turkish German Gynaecological Association 2012 13(3):157-61. [Google Scholar]
[8]. Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K, Kato H, Effects of Clomiphenecitrate on the endometrial thickness and echogenic pattern of the endometriumFertility and Sterility 1997 67(2):256-60. [Google Scholar]
[9]. Palomba S, Russo T, Orio F, Falbo A, Manguso F, Sammartino A, Uterine effects of clomiphene citrate in women with polycystic ovary syndrome: a prospective controlled studyHuman Reproduction 2006 21(11):2823-29. [Google Scholar]
[10]. Favaedi M, Taheripanah R, Kabir salmani M, Evaluation of endometrial morphology by scanning electron microscopy on the clomiphene citrate comparison with clomiphene citrate plus Estradiol Valerate and clomiphene plus progesterone in infertile womenInternational journal of reproductive biomedicine 2009 7(2) [Google Scholar]
[11]. Gerli S, Gholami H, Manna A, Scotto Di Frega A, Vitiello C, Unfer V, Use of ethinyl estradiol to reverse the antiestrogenic effects of Clomiphenecitrate in patients undergoing intrauterine insemination: a comparative, randomized studyFertility and Sterility 2000 73(1):85-89. [Google Scholar]
[12]. Shaikh S, Abro S, Shaikh A, Abbasi A, Role of Clomiphene citrate in unexplained infertility at CMC LarkanaMedical Channel 2016 16(1):69-71. [Google Scholar]
[13]. Satirapod C, Wingprawat S, Jultanmas R, Rattanasiri S, Jirawatnotai S, Choktanasiri W, Effect of estradiol valerate on endometrium thickness during clomiphene citrate-stimulated ovulationJ Obstet Gynaecol Res 2013 40(1):96-101. [Google Scholar]
[14]. Unfer V, Costabile L, Gerli S, Papaleo E, Marelli G, Di Renzo G, Low Dose of Ethinyl Estradiol Can Reverse the Antiestrogenic Effects of ClomipheneCitrate on EndometriumGynaecologic and Obstetric Investigation 2001 51(2):120-23. [Google Scholar]
[15]. Yagel S, Ben-Chetrit A, Anteby E, Zacut D, Hochner-Celnikier D, Ron M, The effect of ethinyl estradiol on endometrial thickness and uterine volume during ovulation induction by clomiphene citrateFertil Steril 1992 57:33-36. [Google Scholar]
[16]. Khanna SB, Kaul U, Effects of exogenous ethinyl estradiol on endometrial receptivity in clomipheneinduced cycles in infertile women with polycystic ovaries. effects of exogenous ethinyl estradiol on endometrial receptivity in clomiphene induced cycles in infertile women with polycystic ovariesJournal of Medical Education and Research 2005 7(3):140-45. [Google Scholar]
[17]. Khadem N, Rafie Zadeh Z, Effect of double IUI on pregnancy rate in Superovulated cycles with clomiphene citrateJ Reprod Infertil 2003 4(4):314-21. [Google Scholar]
[18]. Sohrabvand F, Ansari S, Bagheri M, Efficacy of combined metformin–Letrozole in comparison with metformin–Clomiphenecitrate in clomiphene-resistant infertile women with polycystic ovarian diseaseHuman Reproduction 2006 21(6):1432-35. [Google Scholar]
[19]. Seyedoshohadaei F, Zandvakily F, Shahgeibi S, Comparison of the effectiveness of Clomiphenecitrate, tamoxifen and Letrozole in ovulation induction in infertility due to isolated unovulationIran J Reprod Med 2012 10(6):531-36. [Google Scholar]
[20]. Requena A, Herrero J, Landeras J, Navarro E, Neyro J, Salvador C, Use of letrozole in assisted reproduction: a systematic review and meta-analysisHuman Reproduction Update 2008 14(6):571-82. [Google Scholar]
[21]. Bedaiwy MA, Abdelaleem MA, Hussein M, Mousa N, Brunengraber LN, Casper RF, Hormonal, follicular and endometrial dynamics in Letrozole-treated versus natural cycles in patients undergoing controlled ovarian stimulationReprod Biol Endocrinol 2011 9(83):1-6. [Google Scholar]
[22]. Kamath M, George K, Letrozole or clomiphene citrate as first line for anovulatory infertility: a debateReprod Biol Endocrinol 2011 9(1):86 [Google Scholar]
[23]. Holzer H, Casper R, Tulandi T, A new era in ovulation inductionFertility and sterility 2006 85(2):277-84. [Google Scholar]
[24]. Topipat C, Choktanasiri W, Jultanmas R, Weerakiet S, Wongkularb A, Rojanasakul A, A comparison of the effects of clomiphenecitrate and the aromatase inhibitor letrozole on superovulation in asian women with normal ovulatory cyclesGynaecological Endocrinology 2008 24(3):145-50. [Google Scholar]
[25]. Atay V, Cam C, Muhcu M, Cam M, Karateke A, Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulationJournal of International Medical Research 2006 34(1):73-76. [Google Scholar]
[26]. Morad A, Elhadi Farag M, Impact of letrozole on ultrasonographic markers of endometrial receptivity in polycystic ovary syndrome women with poor endometrial response to clomiphenecitrate despite adequate ovulationMiddle East Fertility Society Journal 2015 20(3):182-87. [Google Scholar]
[27]. Hendawy SF, Samaha HE, Elkholy MF, Letrozole versus Clomiphenecitrate for Induction of Ovulation in patients with polycystic Ovarian syndrome Undergoing Intrauterine InseminationClinical medicine insights Reproductive health 2011 5:11 [Google Scholar]
[28]. Roy KK, Baruah J, Singla S, Sharma JB, Singh N, Jain SK, A prospective randomized trial comparing the efficacy of Letrozole and Clomiphenecitrate in induction of ovulation in polycystic ovarian syndromeJournal of human reproductive sciences 2012 5(1):20 [Google Scholar]
[29]. Xi W, Liu S, Mao H, Yang Y, Xue X, Lu X, Use of Letrozole and Clomiphenecitrate combined with gonadotropinsin clomiphene-resistant infertile women with polycystic ovary syndrome: a prospective studyDrug design, development and therapy 2015 9:6001 [Google Scholar]
[30]. Sharief M, Nafee NR, Comparison of letrazole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulationJPMA The Journal of the Pakistan Medical Association 2015 65(11):1149-52. [Google Scholar]
[31]. Mitwally MF, Casper RF, Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrateFertil Steril 2001 75:305-09. [Google Scholar]
[32]. Fisher SA, Reid RL, Van Vugt DA, Casper RF, A randomized double-blind comparison of the effects of Clomiphenecitrate and the aromatase inhibitor on ovulatory function in normal womenFertil Steril 2002 78:280-85. [Google Scholar]
[33]. Ghomian N, Khosravi A, Mousavifar N, A randomized clinical trial on comparing the cycle characteristics of two different initiation days of letrozole treatment in clomiphene citrate resistant pcos patients in iui cyclesInt J Fertil Steril 2015 9(1):17-26. [Google Scholar]
[34]. Nahid L, Sirous K, Comparison of the effects of letrozole and clomiphene citrate for ovulation induction in infertile women with polycystic ovary syndromeMinerva Ginecologica 2012 64(3):253-58. [Google Scholar]
[35]. Akbari S, Ayazi Roozbahani M, Ayazi Roozbahani F, Comparing of Letrozole versus clomiphene citrate combined with gonadotropins in intrauterine insemination cyclesIran J Reprod Med 2012 10(1):29-32. [Google Scholar]
[36]. He D, Jiang F, Meta-analysis of Letrozole versus clomiphene citrate in polycystic ovary syndromeReproductive Bio Medicine Online 2011 23(1):91-96. [Google Scholar]