An Incidental Finding of Bilateral Dysgerminoma During Cesarean Section: Dilemmas in Management
Mamta Gupta1, Rita Jindal2, Vandana Saini3
1 Senior Consultant and Head of Department, Department of Obstetrics and Gynaecology, Hindu Rao Hospital, Delhi, India.
2 Specialist, Department of Obstetrics and Gynaecology, Hindu Rao Hospital, Delhi, India.
3 Senior Specialist, Department of Obstetrics and Gynaecology, Hindu Rao Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mamta Gupta, A-18, Greenview Aptt. Sector 9, Rohini, Delhi-110085, India.
E-mail: write2mamta55@gmail.com
Dysgerminoma is an uncommon malignant tumour arising from germ cells of ovary. It occurs mostly in the reproductive age group. Its association with pregnancy is rare. Its management remains a challenge especially in an unsuspected case. We present a case of a woman, aged 28-year-old gravida2 para1 who reported to us at 36 weeks’ pregnancy with severe preeclampsia and previous caesarean section. On ultrasound she was reported as having subserosal fibroids with single live fetus of 35 weeks and 3 days gestation. She delivered a live baby by caesarean section done for failed induction. Intraoperatively bilateral ovarian masses were found and removed which were later confirmed to be dysgerminoma on histopathological examination. As she was not diagnosed dysgerminoma pre-operatively, complete work up i.e., tumour markers and MRI was not done, leading to dilemmas in management. Though standard protocols for management of dysgerminoma with pregnancy exist, yet management of these incidentally diagnosed dysgerminomas remains a dilemma.
Fertility sparing surgery, Germ cells of ovary, Incompletely staged dysgerminomas
Case Report
A 28-year-old lady gravida2 was admitted at 35 weeks and 3 days of gestation with blood pressure of 160/104 mmHg. She had history of previous caesarean section done 3 years back for pre-eclampsia with failed induction. There was no history of bladder, bowel complaints, weight loss and no family history of breast, ovarian or colon malignancy.
On abdominal examination, uterus was 36 weeks size with cephalic presentation. A firm lump of 15X8 cm was felt adjacent to uterus on left side, non tender with restricted mobility. Fetal heart rate was 140/min. Her BP remained high in the range of 160-170 mmHg systolic and 100-110 mm Hg diastolic. Her investigations including complete blood count, platelet count, liver function test, renal function test, serum uric acid and fundus examination were normal. Albuminuria was present.
Ultrasound revealed a single live fetus of 35 weeks and 3 days gestation. Placenta was situated anteriorly in upper uterine segment. Amniotic fluid index was 5. Bilateral hypoechoic lesions were noted of size 15X9 cm and 2.8X 5 cm which were reported as subserosal fibroids. An ultrasound examination that was done for menstrual irregularity prior to the current pregnancy was reported to be normal.
A decision for termination of pregnancy was taken in view of severe pre-eclampsia. She delivered a live healthy baby boy, 2.22 kg by caesarean section which was done for failed induction. Intraoperatively, a large firm ovarian mass with intact capsule and bosselated surface was seen on left side. A smaller ovarian mass was seen in the right adnexa. Bilateral fallopian tubes were stretched and adhered over the masses [Table/Fig-1]. Healthy ovarian tissue was not identified. There was no free fluid.
Bilateral ovarian tumours intraoperatively.
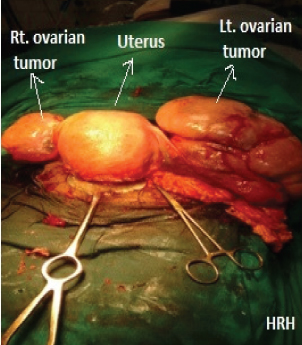
Both masses with fallopian tubes were removed after consent [Table/Fig-2]. No enlarged para-aortic or retroperitoneal lymph nodes were appreciated and omentum was unremarkable. No abnormality or nodularity was observed in any other intra-abdominal organ. Her intra and postoperative period was uneventful.
Bilateral ovarian tumours after excision.
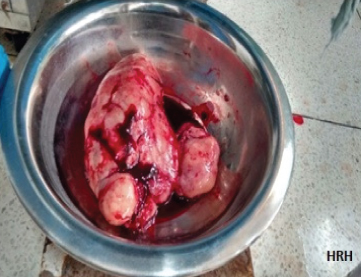
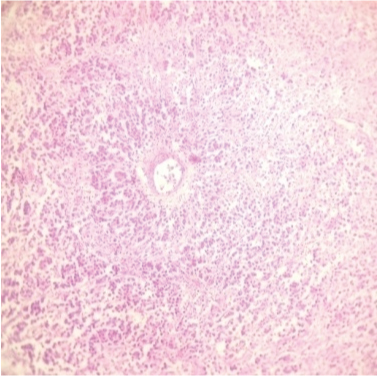
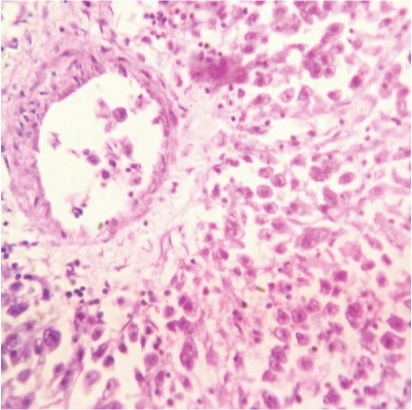
The ovarian masses weighed 1095gm and 125gm respectively. On cut section, the masses were grey-white with cystic changes and haemorrhage. Histopathology unexpectedly revealed bilateral dysgerminoma, showing sheets of tumour cells (with vesicular nuclei) separated by fibrous septa (lymphocytic infiltrate) [Table/Fig-3,4]. Postoperatively tumour markers and Positron Emission Tomography (PET) scan were done and found to be normal. Postoperatively, patient has received 3 cycles of BEP (bleomycin, etoposide and cisplatin) chemotherapy and is doing well.
Histopathology showing sheets of tumour cells, separated by fibrous septa (100x, H&E stain).
Microsection showing tumour cells with vesicular nuclei. Fibrous septa showing lymphocytic infiltrate (400x, H&E stain).
Discussion
Ovarian Germ Cell Tumours (GCTs) comprise 27.1% of all ovarian cancers [1]. Only 3% are malignant, dysgerminomas, being the most common. This case needs reporting and discussion because management of incidentally diagnosed tumour is challenging. The patient was not worked up pre-operatively for ovarian tumour (markers and MRI) as antenatally, ultrasound reported it to be a sub-serosal uterine fibroid. Dysgerminoma of ovary occurs in reproductive age usually below 30 years and can affect fertility and pregnancy outcome. Association of dysgerminoma with pregnancy is rarely reported (0.9%) [2]. Most of the dysgerminomas in pregnancy are unilateral [3]. Bilateral dysgerminomas as seen in our case are uncommon and are found in 15-20% cases only.
These tumours may be asymptomatic or can present with pain in abdomen, misdiagnosed as acute appendicitis, ectopic pregnancy, acute abdomen, distension of abdomen with bleeding per vaginum [4]. It can be misdiagnosed as fibroid [5] as in our case. These tumours can affect conception and if pregnancy occurs, it can lead to fetomaternal compromise. Increased risk of torsion, incarceration, rupture and haemorrhage can occur during pregnancy and vaginal delivery. Fetal demise has been reported to be in 25% of cases [6]. Spontaneous successful pregnancy with no feto-maternal compromise has been reported as occured in our case [2].
Values of tumour markers are misleading in pregnancy and should be interpreted with caution. Serum beta human chorionic gonadotropin (hCG) and lactate dehydrogenase (LDH) rise in pregnancy. Management of these tumours is challenging. Future fertility, effect of radiotherapy and chemotherapy on the developing fetus, stage of tumour, its high sensitivity to radiotherapy and chemotherapy are the factors which should be considered before individualizing treatment. Most of the adnexal masses in 1st trimester of pregnancy resolve spontaneously. However, if suspicious of malignancy then termination can be offered to women not desirous of pregnancy, followed by total abdominal hysterectomy with bilateral salpingo-oophrectomy and surgical staging [7].
If pregnancy is desired, ideal time for intervention is 16-18 weeks of gestation with removal of tumour and surgical staging. Chemotherapy can be given after surgery, though feto-maternal complications i.e., abortions, haemopoietic depression, infections, fetal pancytopenia, delayed cognitive development and low birth weight can occur [8].
In stage 1A, conservative or fertility sparing surgery [9] can be done if patient is desirous of pregnancy and includes- staging laparotomy with unilateral salpingo-oophrectomy. No chemotherapy is required for stage 1A tumours unless recurrence [10] (9.2% cases) occurs. Treatment for stage 1B is bilateral salpingo-oophrectomy with or without total abdominal hysterectomy [3] followed by three cycles of BEP chemotherapy [11] as was done in our case. However, fertility sparing surgery can be done even in bilateral dysgerminomas if patient is desirous of future pregnancy as no difference in outcome between fertility sparing and non conservative surgery has been found [10]. Treatment for stage II, III & IV is complete resection followed by four cycles of BEP chemotherapy [11]. Patients with bulky residual disease require additional cycles.
Radiation is used for periaortic and pelvic lymph node metastasis. It may be used for any dysgerminoma staged IB to III. Second look surgery is not required for dysgerminomas and should be considered if tumour markers are persistently elevated especially if abnormal findings are seen on post-treatment imaging [11].
In incompletely staged/worked up tumours; tumour markers i.e., serum alpha feto-protein (AFP), beta hCG and LDH with CT scan of chest, abdomen and pelvis should be done and if found to be normal, follow-up is done, as in our case. If they are found to be abnormal or become abnormal during follow-up, then laparotomy and staging should be done. Pregnancy should be ruled out in patients who Undergo fertility sparing surgery as they can have elevations in AFP and beta-hCG. Recurrence rate is 20% in unstaged patients [12].
Though the recurrence rates have been reported more in the incompletely staged or unstaged patients [12,13], yet these tumours are highly chemosensitive and prognosis is excellent. Follow-up should be done every 3-4 monthly for 3 years, then 6 monthly for next 2 years and then yearly upto 10 years. CT scan should be done at 6, 12 months especially if markers are negative at diagnosis. Follow- up with serum LDH can be done [14]. The fetomaternal outcome of patients with pregnancy with dysgerminoma is excellent. A 97%-100% survival rate with ovarian dysgerminoma has been reported [15].
Conclusion
Pregnant women with dysgerminoma are young and have good feto maternal outcome. Fertility sparing surgery can be offered in women, desirous of pregnancy. If these tumours are found incidentally during caesarean section, tumour markers and CT scan should be done postoperatively to plan optimum treatment.
[1]. Hashmi AA, Hussain ZF, Bhagwani AR, Edhi MM, Faridi N, Hussain SD, Clinicopathologic features of ovarian neoplasms with emphasis on borderline ovarian tumours: an institutional perspectiveBMC Res Notes 2016 9(1):205 [Google Scholar]
[2]. Ueda M, Ueki M, Ovarian tumours associated with pregnancyInt J Gynaecol Obstet 1996 55(1):59-65. [Google Scholar]
[3]. Vicus D, Beiner ME, Klachook S, Le L, Laframboise S, Mackay H, Pure dysgerminoma of the ovary 35 years on: a single institutional experienceGynecol Oncol 2010 117(1):23-26. [Google Scholar]
[4]. Jain M, Budhwani C, Jain AK, Hazari RA, Pregnancy with Ovarian dysgerminoma: An unusual diagnosisJ of dental and Medical Sciences 2013 11(5):53-57. [Google Scholar]
[5]. Gauza JE, Reberti AG, Silva JC, Pope LZB, da Rocha dos Santos JCR, Quintana SM, Diagnosis of ovarian dysgerminoma during pregnancyRev Assoc Med Bras 2010 56(5):517-19.São Paulo [Google Scholar]
[6]. Karlen JR, Akbari A, Cook WA, Dysgerminoma associated with pregnancyObstet Gynecol 1979 53(3):330-35. [Google Scholar]
[7]. Yakasai IA, Bappa LA, Diagnosis and management of adnexal masses in pregnancyJ Surg Tech Case Rep 2012 4(2):79-85. [Google Scholar]
[8]. Cardonic E, Lacobucci A, Use of chemotherapy during human pregnancyLancet Oncol 2004 5:283 [Google Scholar]
[9]. Zanetta G, Bonnazi C, Cantu M, Binidagger S, Locatelli A, Bretina G, Survival and reproductive function after treatment of malignant germ cell ovarian tumoursJ Clin Oncol 2001 19(4):1015-20. [Google Scholar]
[10]. Husaini AL, Soudy H, ElDin Darwish A, Ahmed M, Eltigani A, Mubarak AL, Pure dysgerminoma of the ovary: a single institutional experience of 65 patientsMed Oncol 2012 29(4):2944-48. [Google Scholar]
[11]. Pectasides D, Pectasides E, Kassanos D, Germ cell tumours of the ovaryCancer Treat Rev 2008 34(5):427-41. [Google Scholar]
[12]. Mangili G, Sigismondi C, Lorusso Cormio G, Scollo P, Viganä R, Is surgical restaging indicated in apparent stage IA pure ovarian dysgerminoma? The MITO group retrospective experienceGynecol Oncol 2011 121(2):280-84. [Google Scholar]
[13]. Chauhan A, Desai A, Kapadia A, Patel S, Garg S, Dave K, Pure dysgerminoma of ovary: 11 years experienceJ Obstet Gynecol Ind 2004 54(2):162-66. [Google Scholar]
[14]. Michener CM, Wu AY. Ovarian Dysgerminomas. Warner K Huh, 2015 available from http://emedicine.medscape.com/article/253701-overview [Google Scholar]
[15]. Solheim O, Gershenson DM, Trope CG, Rokkones E, Sun CC, Weedon-Fekjaer H, Prognostic factors in malignant ovarian germ cell tumours (The Surveillance, Epidemiology and End Results experience 1978-2010)Eur J Cancer 2014 50(11):1942-50. [Google Scholar]