Acute cholecystitis is a commonly encountered condition in surgical emergencies it is rarely associated with Gall Bladder (GB) perforation as a complication. Urgent surgical intervention is often needed to reduce serious morbidity and mortality [1]. An essential role is played by imaging because perforation itself does not produce any specific signs and symptoms to differentiate it from uncomplicated acute cholecystitis. The perforation time can also be highly variable ranging from as early as few days to several weeks from the time of first onset of inflammation. A correct decision making in order to diagnose this insidious complication often requires use of multiple imaging modalities [2,3]. While sonography is the first line of investigation used in most centres, cross-sectional imaging is increasingly advocated to establish an early diagnosis before making necessary intervention. An incidence of 2 to 11% of perforation cases had been reported in literature [1]. Undoubtedly, GB perforation remains a diagnostic challenge due to the high mortality associated with a delay in the correct judgement and intervention and the variability in accuracy of different imaging modalities [1,3]. The purpose of this study was to highlight the role of cross-sectional imagings in early diagnosis of GB perforation and compare the diagnostic accuracy of Ultrasonography (USG) with other cross-sectional imagings.
Materials and Methods
The cross-sectional images in 17 patients with GB perforation were collected retrospectively over a period of 1 year from January 2015 to December 2015. Patients, whose clinical details raised possibility of acute cholecystitis or possible perforation, were included and where at least a cross-sectional imaging (either CT or MRI) was available following the baseline ultrasound report. Those cases where no cross-sectional imaging data or sonography reports were available were excluded from the study. Patient details have been anonymised and consent was taken from the ethical committee. The cross-sectional images were reviewed retrospectively by two radiologists who were blinded with regard to the earlier sonographic diagnosis.
USG was conducted in Seimens ACUSON antares ultrasound) (Siemens Medical Systems, Erlangen, Germany) using curved (2-6.67 MHz) and linear (5-13 MHz) array probes. Sonography reports, used as the baseline investigation for all cases of GB perforation were correlated with CT / MRI findings and final operative findings. In 14 patients CT scan was done in 64 slice MDCT scanner, (Aquilion 64, Toshiba Medical Systems Corporation). In 4 patients MRI scan were done using Siemens Avantos 1.5 Tesla machine. Among these 4 patients two of them were referred for a MRI instead of a CT because they had accompanying symptoms of possible biliary pathology or cholecystoenteric fistula, one patient had deranged renal function tests so was not an appropriate candidate for a contrast CT. Furthermore, in only one patient the final fistuluous communication between GB and adjacent duodenum could only be established after a MRI was performed as CT scan result was indeterminate. We evaluated the cross-sectional findings of GB perforation, GB wall defect, GB wall thickening, Cholelithiasis, pericholecystic collection, intra-hepatic abscess, focal bulging of GB wall, streaky omentum or mesenteric fatty thickening and compared with the baseline ultrasound findings. For the significance of comparison between ultrasound and Cross-sectional imaging (CT/MRI) we compared the most specific sign (GB wall defect) in relation to the size of the defects [1,4]. Niemeier’s age old classification carries prognostic significance with regard to the outcome of the patient, hence appropriate preoperative categorisation by a particular imaging modality according to Niemeier’s classification was used as second parameter [5].
Results
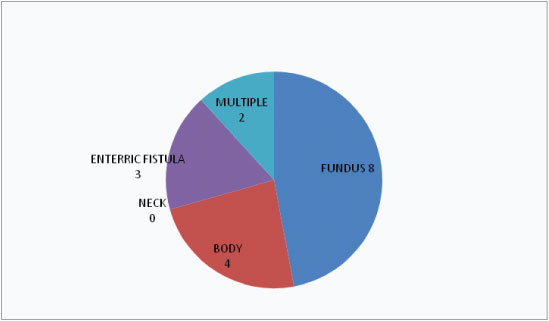
In our study cases females (76%) outnumbered males (24%). Their age ranged from 30-84 years (mean 56 years). Among the 17 cases, fundal perforation cases were maximum (8) followed by body as the second commonest site of GB perforation [Table/Fig-1]. GB wall defect was taken as the most definitive criteria for diagnosing perforations on both imaging modalities. Based on final operative findings we have classified the size of GB wall defect in three categories size1(< 5 mm), size 2(> =5 and < 10 mm) and size 3(>10 mm). Among our cases maximum were in size 3 with a rent defect more than 10 mm (9) followed by size 1 (6) and only 2 cases with GB wall defect measuring between 5-10 mm [Table/Fig-2]. Again based on the clinical presentation and operative findings, each patient of Gall Bladder perforation have been categorised in to Niemeier’s: I(acute); II(sub acute); and III(chronic). Thus, the numbers I, II and III denote the respective Niemeier’s classification according to USG, cross-sectional imaging and operative findings while the number of cases in both modalities which could not be pre operatively diagnosed by imaging have been assigned under “0” subheading. In our series, we had maximum cases of Niemeier’s type II or sub acute perforations (12) followed by type III or chronic (4) and there was only one case of type I or acute perforation. [Tables/Fig-3,4] determine the pre operative classification of the perforation cases based on USG and CT/MRI findings respectively with corresponding size of the perforations. From [Table/Fig-3] we see that USG could diagnose 9 out of 17 cases all of which had a rent defect of >10 mm and thus a significant association (p-value=0.002) was found between the rent size and percentage of positive cases detected by USG. Simultaneously this table shows that USG failed to diagnose any of the chronic/type III perforations which presented with cholecysto enteric fistula formation and those of the type II cases who had a rent defect of less than 10 mm. While CT/MRI [Table/Fig-4] had a much less number of negative results (3 out of 17=17.6%) compared to USG (8 out of 17=47%). CT/MRI could correctly diagnose 3 out of 4 cases of type III/chronic perforations with fistulous communication with the bowel loops and 8 out of 10 cases of type II/sub acute perforations [Table/Fig-4]. However, even with cross-sectional imaging the chances of getting a correct diagnosis increased with the size of the defect with significant association (p-value=0.008). Finally we have assessed the percentage agreement of preoperative findings of USG and CT/MRI with actual per operative findings in [Table/Fig-5,6] respectively. Thus from these tables we can see that there was a higher percentage of agreement between CT/MRI and operative findings (82.37%, Kappa score -0.54) compared to USG and operative results (62.7%, Kappa score -0.39). A fair agreement (74.5%) with a kappa score of 0.45 approximately was also found between the imaging modalities USG and CT/MRI [Table/Fig-7] in categorising GB perforations in accordance with Niemeier’s classification.
Location of perforation defect.
GB wall defect size among males and females.
| GB Wall Defect Size | Females | Males | Total |
|---|
| Size of Defect Size 1(<5 Mm) | 4 | 2 | 6 |
| Size 2(5-10 Mm) | 1 | 1 | 2 |
| Size 3(> 10 Mm) | 8 | 1 | 9 |
| Total | 13 | 4 | 17 |
Niemeier’s classification made according to preoperative ultrasound.
| GB wall defect size | Type I | Type II | Negative results on USG | Total |
|---|
| Size 1 | 0 | 0 | 6 | 6 |
| Size 2 | 0 | 0 | 2 | 2 |
| Size 3 | 1 | 8 | 0 | 9 |
| Total | 1 | 8 | 8 | 17 |
There were no Type III perforations detected by USG.
Pearson chi 2(4)=17.00, p =0.002
Niemeier’s classification made according to preoperative CT/MRI.
| GB wall defect size | Type i | Type ii | Type iii | Negative results on CT/MRI | Total |
|---|
| Size 1 | 0 | 0 | 3 | 3 | 6 |
| Size 2 | 0 | 2 | 0 | 0 | 2 |
| Size 3 | 1 | 8 | 0 | 0 | 9 |
| Total | 1 | 10 | 3 | 3 | 17 |
Pearson chi 2(6)=17.37, p =0.008
Niemeier’s Classification made based on operative findings.
| USG | Type I | Type II | Type III | Total |
|---|
| USG Classified Type I | 1 | 0 | 0 | 1 |
| USG Classified Type II | 0 | 8 | 0 | 8 |
| Negative on USG | 0 | 4 | 4 | 8 |
| Total | 1 | 12 | 4 | 17 |
No Type III perforations detected by USG
Niemeier’s Classification made based on operative findings.
| CT/MRI | Type i | Type ii | Type iii | Total |
|---|
| CT/MRI Classified Type I | 1 | 0 | 0 | 1 |
| CT/MRI Classified Type II | 0 | 10 | 0 | 10 |
| CT/MRI Classified Type III | 0 | 0 | 3 | 3 |
| Negative on CT/MRI | 0 | 2 | 1 | 3 |
| Total | 1 | 12 | 4 | 17 |
CT/MRI and operative findings compared to USG.
| Niemeier’s classification by | Preoperative CT/MRI |
|---|
| Preoperative USG | Type I | Type II | Type III | Negative | Total |
|---|
| Classified Type I | 1 | 0 | 0 | 0 | 1 |
| Classified Type II | 0 | 7 | 1 | 0 | 8 |
| Negative | 0 | 3 | 2 | 3 | 8 |
| Total | 1 | 10 | 3 | 3 | 17 |
Discussion
GB Perforation usually occurs as a result of necrotic damage of GB wall due to ischemia, infection of Rokitansky–Aschoff sinuses and/or pressure necrosis of impacted gallstones [6,7]. GB perforation is typically seen in the setting of diseased GB such as cholecystitis, malignancy and corticosteroid use and vascular compromise. The reported pathomechanism of GB perforation is considered to be GB distention due to impaction of a calculus in the cystic duct, followed by secondary vascular impairment and ischemia of the GB wall [8]. As a result, the fundus, which is the least well-vascularized part of the GB, is the most common site of perforation [7,8].
Many clinical studies have reported a high incidence of GB perforation after acute cholecystitis, leading frequently to peritonitis and sepsis in diabetic patients and its mortality rate is closely related to delay in diagnosis [9,10]. Thus, early diagnosis of GB perforation is absolutely necessary, especially in diabetic patients.
Though the incidence of acute uncomplicated cholecystitis had been reported to be more among females (female to male ratio of 2:1), GB perforation is more frequent in males [10,11]. Martin et al., also mentions a higher chances of perforation among elderly patients [11].
In 1934, Niemeier proposed a classification for GB perforation that is still used today and has prognostic implications [12]: Type I, acute free perforation of the GB into the peritoneal cavity without protective adhesions; Type II, sub acute perforation surrounded by a pericholecystic abscess walled off by adhesions; and Type III, chronic perforation with presence of a fistulous communication between GB and a viscus. Subacute (type II) perforations are the most common type in most reported series [12] accounting for 60% of all cases; chronic (type III) perforation seen in 30% and acute perforation (type I) in 10%. Type I perforations usually carry a higher mortality. It has been acknowledged from previous literature that Type III GB perforation cases have high association with long history of gall stones [11,12]. In all four of our patients with type III perforations there was a long history of cholelithiasis. Fletcher et al., reported 40% mortality for type I, 4% mortality for type II and no mortality for type III perforations [13]. According to Roslyn et al., type I and II GB perforation are more often seen in patients around 50 years [14], whereas type III GB perforations are common in more aged population. However, we did not make similar observations among our patients with three out of four patients of type III perforations had age less than 50 years.
GB perforation often presents a diagnostic challenge. Clinical signs and symptoms depend on the etiology and are usually nonspecific. They can range from right upper quadrant to generalized abdominal pain, tenderness, rigidity, and guarding (signs of peritonitis) to nonspecific abdominal symptoms (nausea, vomiting, vague upper abdominal discomfort, or pain). Often it is difficult to differentiate GB perforation from uncomplicated acute cholecystitis likely because bile leak from the perforation might be contained [8,10,15]. Complications of GB perforations include bile leak and peritonitis; abscesses around the GB fossa, intraperitoneal or intrahepatic; intraperitoneal air; sepsis or septic shock; fistulae; and bowel obstruction [15].
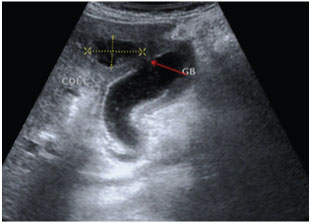
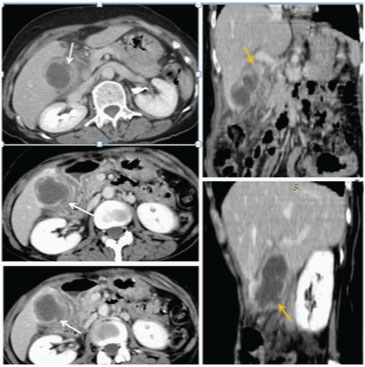
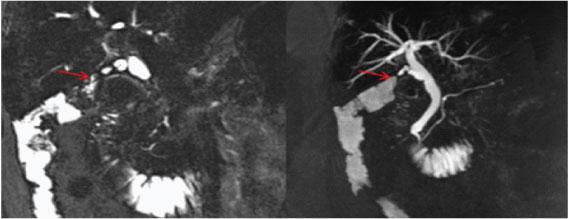
In most institutes Sonography is the first investigation recommended for a patient presenting in ER with signs of cholecystitis. As most patients with a diagnosis of uncomplicated cholecystitis are rarely advised a cross-sectional imaging and treated conservatively followed by an elective cholecystectomy, hence making a diagnosis of perforation on USG and segregating such cases who require urgent intervention is of paramount importance [16,17]. USG is usually the initial method of investigation of choice in patients though findings may be non-specific. US findings in acute cholecystitis, such as the GB wall thickening, GB distension, pericholecystic free fluid, and positive sonographic Murphy sign, may also be present in GB perforation cases [16,17]. Chau et al., first described the “sonographic-hole” sign, which is the direct visualization of a GB wall defect on USG [Table/Fig-8]. It is a very specific sign of GB perforation that can be seen on USG, CT or MR Imaging and hence used in our study as a parameter to compare both the modalities [16]. We saw the hole sign in 9 out of 17 cases in USG as compared to CT/MRI scan which obviously had a better sensitivity(14 out of 17). Our findings corroborates with Kim et al., who also reported a higher sensitivity of CT compared to USG in picking perforations in GB wall [18]. The focal rent/discontinuity in the GB wall was better appreciated on CT/MRI [Table/Fig-9], secondly extra luminal gall stones observed in few cases [Table/Fig-10] were also specific for perforations. Besides associated complications of GB perforations like peri-cholecystic collections, mostly associated with type II perforations were also better demonstrated on cross-sectional imaging [19–21]. The relatively poor sensitivity of ultrasound in detecting dehiscence in GB could be attributed to several factors like large body habitus of the patient, acoustic shadow from stones obscuring the dependent wall, artefacts due to pneumobilia. In type III or chronic perforations USG failed to demonstrate the fistulous communication because the fistulous communications were small and difficult enough to be detected, besides the reverberation artifact from air in the bowel loop at the fistulous site reduced the exclusion value of Ultrasound. MRI with heavily T2W sequences [22] was particularly useful for depicting the fistulous tract [Table/Fig-11]. Spilled gall stones which could only be seen by cross-sectional imaging [Table/Fig-10] should always be tried to remove on surgery as they may incite inflammation and erode into gut [19,23].
A rent(red arrow) was seen near the fundus with a pericholecystic collection with direct continuity into the GB.
Axial post contrast CT scan images show inflamed and oedematous walls with pericholecystic inflammation. Multiple variable sizes GB wall perforations were in GB fundus and body regions. (white arrows). Corona and Sagittal reformatted images also show multi-sites GB perforations.
Coronal and Axial T2W MRI images in different patients show extra luminal gall stones (red arrows).
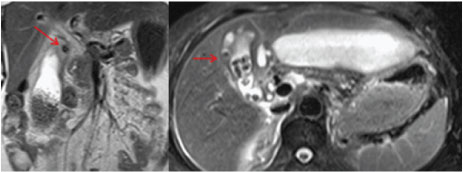
HASTE MRCP image well demonstrates fistulous communication between the contracted GB and hepatic flexure of colon (red arrows).
In our study population, single patient with type I or acute perforation was managed by cholecystostomy with abdominal drain placement, however for all other patients with subacute or type II perforations laparoscopic and open cholecystectomy were carried out as standard procedures. A patient of type III perforations who presented with gall stone ileus was managed by emergency laparotomy. Other type III perforation cases were treated by repair of fistula with cholecystectomy. The patient with type I and two patients of subacute or type II perforations with associated extensive peri cholecystic abscesses ultimately succumbed to peritonitis.
Considering our limited study population we did not try to find the parameters validating these tests rather we tried to determine the magnitude of agreement between the diagnostic tests and operative findings. From [Table/Fig-5,6 and 7] we found a significant association between the size of GB wall defect and the probability of a correct diagnosis by pre operative imaging with cross-sectional imaging definitely surpassing ultrasound in determining positive cases. A higher agreement was also found between cross-sectional imaging and peroperative findings in classifying perforations according to Niemeier’s classification similar to other studies in the past [18–20].
Limitation
Only 17 patients were included in the sample and no control population was included to assess the actual specificity and accuracy of these tests. We look forward to do a prospective study in future including a larger sample size.
Conclusion
In cases with high index of clinical suspicion for perforation or those with an indeterminate or difficult ultrasound scan, the idea is to keep imaging and cross-sectional imaging should always be recommended. While cross-sectional imaging undoubtedly scored better than ultrasound in detecting perforations, in acute (typeI) and subacute (type II) cases CT should be the ideal investigation following ultrasound whereas in chronic cases with long standing symptoms and possible fistulous communication MRI would be better for confirmation. Early institution of one or more cross-sectional imaging modality may establish a prompt definitive diagnosis of GB perforation and hence decrease morbidity and mortality.
There were no Type III perforations detected by USG.
Pearson chi 2(4)=17.00, p =0.002
Pearson chi 2(6)=17.37, p =0.008
No Type III perforations detected by USG