Invasive Pulmonary Aspergillosis (IPA) is an important opportunistic infection in critically ill patients with various underlying risk factors and is associated with a high degree of morbidity and mortality [1]. The incidence of this condition has increased in recent years. Incidence and mortality is high especially among patients with neutropenia and haematopoietic stem cell transplant recipients [1]. The other risk factors include major immunodeficiency conditions like advanced AIDS, prolonged high-dose corticosteroid and cytotoxic therapy and Chronic Granulomatous Disease (CGD).
As per the latest European Organisation for Research and Treatment of Cancer (EORTC) guidelines [2] the diagnosis of proven IPA is by histopathological examination and or culture of Aspergillus species from lung tissue. However, the invasive nature of obtaining sterile tissue limits the utility of this criterion in severely sick and debilitated patients.
Diagnosis based on imaging techniques like High Resolution Computer Tomography (HRCT) and Magnetic Resonance Imaging (MRI) is useful, but not performed routinely in resource limited settings. Serological test like Galactomannan assay (GM assay)which detects the cellular antigens secreted by Aspergillus is an attractive option for the diagnosis of IPA as it is less invasive and aids in the early diagnosis of the disease. The assay can give a positive result much earlier than the imaging studies or culture results [1].
Galactomannan, a polysaccharide cell wall component released into the serum during the growth phase of Aspergillus is detected by a double-sandwich ELISA method, the GM assay test [3]. Serum GM assay has been in use for the diagnosis of this condition since 2008. Though the EORTC study group has established guidelines for the diagnosis of IPA using GM assay, these guidelines are found to be reliable only when applied to patients with specific host factors [2]. There is limited data on the utility and performance characteristics of this assay to diagnose Invasive Pulmonary Aspergillosis (IPA) in patients with underlying co-morbidities other than those recommended by the EORTC guidelines. The performance characteristics of this assay vary with the cut-offs used. Pfeiffer in his meta-analysis has reported a sensitivity, specificity, PPV and NPV value of 71%, 89%, 25-62% and 92-98% for GM assay using 0.5 as the galactomannan index (GI) cut-off whereas SA Sarraffzadeh et al., has reported the same parameters to be 69.2%, 72.2%, 47.4%, 86.7% using 1.5 as the cut-off [4,5]. Lack of unanimous consensus regarding the optimum cut-off to be used and the reproducibility of assay results in an endemic country like India in a mixed patient population is a concern.
Though a few reports of this assay have been published from India (Khanna et al.,) since 2013, none of them have addressed the issues of validation of the kit cut-off using a healthy control group which could directly affect the diagnostic cut- off to be used as well as performance of the kit in a mixed patient population [6]. This study was therefore undertaken to establish an in-house cut-off and compare the utility of this with the kit cut-off (0.5) to diagnose and categorize IPA as ‘proven’, ‘probable’ and ‘possible’ in patients with varied underlying risk factors.
Materials and Methods
This observational study was undertaken from January 2013 - December 2014 in the Department of Microbiology, St. John’s Medical College and Hospital, Bangalore, Karnataka, India which is a 1200 bed multi-specialty tertiary care teaching hospital. This study was approved by the Institutional Ethics Committee.
A total of 50 serum samples (2ml serum separated from 5ml of blood from both patients and controls), 25 each from healthy laboratory workers and patients admitted to the hospital (screened out of the 100 cases) with a clinical diagnosis of IPA (23 adults and 2 paediatric) were analyzed. All the patients presented with symptoms of cough and dyspnoea. A total of 15 (60%) of them had moderate to severe haemoptysis and pleuritic chest pain. A total of 17 of the 25 (68%) patients had underlying co-morbidities like uncontrolled diabetes mellitus in 13 (52%), liver carcinoma (1), leukaemia (2) and HIV (1). All the patients included in the study were on regular follow-up for their underlying co-morbid conditions at our hospital. With a presumptive diagnosis of IPA at the time of presentation, they were admitted for further management. Histopathological, microbiological and radiological investigations were carried out as feasible. The serum samples received from these patients for other microbiological investigations stored at -70°C over a period of 18 months were used to perform GM assay. Prior to the availability of GM results the patients were categorized as proven (3), probable (19) and possible (3) IPA based on all other established clinical, histopathological, microbiological and radiological criteria. The GM assay was performed using Biorad PlateliaTM Aspergillus Kit as per the manufacturer’s instructions.
Serum aliquots in sterile vials were pretreated with serum treatment solution provided by the manufacturer. As per kit instructions the samples were kept in the boiling water bath for 4-5 minutes and then centrifuged at 10000 g to dissociate immune – complexes and precipitate the proteins that can interfere with the test. The supernatants were collected and labeled. The kit procedure was followed to perform the ELISA using 50μl of the supernatants. The results were read using a spectrophotometer with an Optical density measured at 450 nm.
The in-house cut-off was calculated by plotting the Receiver Operating Characteristic Curve (ROC) using both the patient and control samples. The SPSS version 22 was used for the analysis. Patients were categorized as proven, probable and possible IPA based on the EORTC 2008 guidelines before and after the availability of the GM assay result [2]. Both the in-house as well as kit cut-off (0.5GI) were used for analysis. Clinical and demographic details of the patients were correlated with the assay findings.
Results
Out of 25 patients with a clinical diagnosis of IPA, males 18 (72%) were predominant among the adults and 7 (28%) were female patients. 60% of the patients (15 of 25) included in this study were in the 30 - 50 years age group and 5 were above 50 years. This association between the age group and IPA was found to be statistically significant (p<0.01) [Table/Fig-1].
Clinical and demographic data of patients.
| Characteristics | Percentage |
|---|
| Age |
| <10 | 2(8%) |
| 11-20 | 2(8 %) |
| 21-30 | 1(4%) |
| 31-50 | 15(60%) (p value <0.01) |
| 51-60 | 2(8%) |
| >60 | 3(12%) |
| Sex: |
| Male | 18(72%) |
| Female | 7(28%) |
| Co-Morbidity |
| Diabetes | 13(52%) |
| Solid Tumour | 1(4%) |
| Haematological Malignancy | 2(8%) |
| ICU Stay and Duration |
| YES | 10(40%) |
| NO | 15(60%) |
| <5 | 3(12%) |
| 5-10 days | 4(16%) |
| >10 | 3(12%) |
| Treatment with Anti-Fungals |
| Voriconazole | 7(28%) |
| Posaconazole | 2(8%) |
| Amphotericin B(Conventional) | 9(36%) |
| Amphotericin B(Liposomal) | 1(4%) |
| None | 3(12%) |
| Both | 3(12%) |
| Status After Treatment |
| Improved | 21(95%) |
| Expired | 1(5%) |
| On follow-up | 15/21(71%) |
| Lost to follow-up | 6/21(29%) |
The mean GM index value among the healthy laboratory personnel was 0.46 [Table/Fig-2]. The sensitivity and specificity of the GM assay was calculated for all the control and patient samples by using the respective GM index value. The GM index values of all the samples were in the range of 0.2 - 4.2. The ROC was plotted and the diagnostic accuracy of the test was evaluated and found to be satisfactory with the value of area under the curve being 0.89 [Table/Fig-3]. With this, the optimum in- house OD index was identified to be 0.52. Using the in-house and the kit cut-offs the sensitivity, specificity, PPV and the NPV were found to be 75%, 79%, 76%, 82% and 79%, 71%, 77% and 82% respectively [Table/Fig-2].
The test parameters at the kit cut off and the in-house cut-off.
| HealthyControls(25) | Patients(25) | Sensitivity | Specificity | PPV | NPV |
|---|
| Mean GIindex | 0.46 | 0.56 | | | | |
| GM positive(in house cutoff) | 6 | 19 | 75% | 79% | 76% | 82% |
| GM positive(kit cutoff) | 6 | 20 | 79% | 71% | 77% | 82% |
Receiver operating characteristic curve for the galactomannan assay.
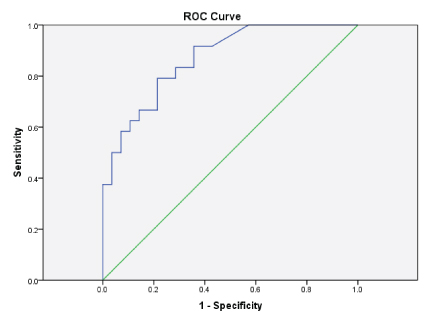
The mean GI of Proven (3), Probable (19), Possible (3) cases as categorized prior to performance of the test were 2.05, 0.73 and 0.58 respectively. The sensitivity for proven, probable and possible cases at the in-house cut-off was 92%, 74%, and 63% respectively and the specificity was found to be 100%, 78% and 58%. Analysing the in–house and kit cut-off to diagnose and categorize patients clinically diagnosed as IPA based on EORTC guidelines without the GA levels, revealed that 19 (3 proven, 14 probable and 2 possible) of the 25 (76%) patients were positive for GM assay using the in–house cut-off of 0.52 and 20 (3 proven, 15 probable and 2 possible) of the 25 (80%) patients were positive for GM assay using the kit cut-off of 0.5. The difference was not found to be statistically significant [Table/Fig-4].
Categorization of the cases prior to the availability of GM assay results.
| Categorizationbefore the test(25) | PositiveTestResults | Re-categorizationafter the availabilityof test result (25) |
|---|
| In housecut-off(19/25) | Kit cut –off (20/25) |
|---|
| Proven | 3 | 3 | 3 | 3 |
| Probable | 19 | 14 | 15 | 21 |
| Possible | 3 | 2 | 2 | 1 |
Discussion
Demonstration of hyphae in histopathologic sample or culture of Aspergillus species from sterile direct specimen (lung biopsy) is considered to be the gold standard for the diagnosis of proven IPA [1]. However, fulfilling either of these criteria is hampered by the invasive nature of the procedure to obtain the specimens especially from high risk immuno-suppressed patients and prolonged turnaround time for the availability of results of both histopathologic examination as well as culture. In this scenario the utility of a non invasive simple ELISA based GM assay for detection of the circulating GM antigen in the serum offering a quick and reliable result gains importance.
Validation of any new test is absolutely essential before it is introduced into routine diagnostic service, especially if it involves reporting of results based on established kit cut-offs. Many factors of both infective and non- infective etiology are known to influence the GM levels in serum. Invasive infections due to Fusarium species, Scedosporium species and Histoplasma can give a false positive GM assay result. It is important to note that these fungi are implicated as invasive pathogens among the same patient population as those predisposed to invasive aspergillosis [1]. Cross-reactivity of galactoxylomannan epitopes with Aspergillus galactomannan can influence GM assay results even in patients with Cryptococcosis [7].
It has also been reported that lipoteichoic acids associated with the cell wall of Bifidobacterium species can bind to the monoclonal antibody used in the GM ELISA resulting in false positivity especially in children and neonates [8]. GM assay can also be elevated in patients receiving intravenous antibiotics such as piperacillin - tazobactam and amoxycillin – clavulanic acid combination [9]. As per the kit literature and few published case reports, false positive GM results may be associated with diets rich in rice, pasta and panned vegetables [10,11]. Knowing that our diet is predominantly rice based and use of antibiotics without prescription is common in our country, we included healthy laboratory personnel with no exposure to antibiotics in the last two months as control group to establish the optimum in house cut-off.
In our study, the GM index in all healthy controls except three was below 0.5. This was in contradiction to our assumption that the baseline GM index could be high due to reasons such as the diet as well as environmental exposure to Aspergillus spores especially in our campus where a lot of construction work is in progress for the past couple of years. The three false positive values were 0.9, 0.73 and 0.54. We could not establish any contributing factors other than diet in these controls including absence of history suggestive of allergic airway disease which could be of fungal etiology. The mean GM index value among the healthy laboratory personnel including and excluding the false positive results was 0.46 and 0.45 respectively. This almost matched the kit cut-off 0.5. The mean GM index of the patient group was 0.56. The sensitivity and specificity of the GM assay was calculated for all the patient and control samples by using the respective GM index value. The GM index values of the healthy controls and patients was in the range of 0.2 – 0.9 and 0.3- 4.2 respectively.
Prior to the availability of the GM assay results, the patients were categorized as proven (3), probable (19) and possible (3) IPA. All the three patients categorized as proven IPA had lung tissue revealing septate fungal hyphae on histopathological or KOH calcofluor examination. The host factors associated with the 19 probable cases included prolonged use of corticosteroids in 10, neutropenia in 3 (associated leukaemia in 2 and transient neutropenia 1), inherited severe immuno deficiency in 2 and treatment with immunosuppressants other than steroids in 4.
According to the EORTC guidelines the LRT fungal disease especially IPA is diagnosed based on the classical CT findings such as the halo sign which is seen as a macronodule (1 cm in diameter) surrounded by a perimeter of ground-glass opacity. It has been reported that the initiation of antifungal treatment based on this finding can result in better outcomes [12]. Unfortunately cost being a major limiting factor in resource constrained settings like ours CT is not routinely performed. The physicians rely more on non- resolving X-ray findings and therefore this was included as a clinical criterion along with CT findings whenever available to categorize our patients. CT was performed in only two of the 19 patients and findings were suggestive of IPA in both. 15 of them showed chest X-ray features suggestive of LRTI such as non- resolving consolidation even with adequate antibiotic therapy for two weeks. One each had sino- nasal infection (X-ray feature) and signs of tracheo bronchitis (bronchoscopic features). All the 19 patients yielded Aspergillus species from any one of the respiratory samples on culture (sputum 8, BAL 10 and nasal aspirate 1). All 3 possible cases had associated host and clinical criteria but no mycological criteria.
A wide range of sensitivity and specificity using different cut-off values have been reported in studies done worldwide. The initial positive cut-off 1.5 for GA recommended by Bio-Rad was modified to ≥0.5 for positive result [13]. Pfeiffer in his meta-analysis reports a sensitivity, specificity, PPV and NPV value of 71%, 89%, 25-62% and 92-98% using 0.5 as the cut-off whereas SA Sarraffzadeh et al., reported 69.2%, 72.2%, 47.4%, 86.7% using 1.5 as the cut-off [4,5]. In this study the sensitivity, specificity, PPV and the NPV using the in-house (0.52) and the kit (0.5) cut-offs did not vary (75%, 79%, 76%, 82% and 79%, 71%, 77% and 82%) and are in concordance with other studies for all parameters except the NPV. This could be attributed to the inclusion of patients with all underlying risk factors in this study [4,5].
Five of the six cases missed in the GM assay using the in–house cut-off were in the probable group. All these patients were still categorized as probable even with the availability of GA as they fulfilled other criteria which included clinical findings and positive Aspergillus species culture from respiratory samples like sputum or BAL. Only one patient of the possible group was missed using either the in-house or kit cut-off. Two of these three patients could therefore be re-categorized as probable using the GM assay results with either of the cut-off [Table/Fig-4].
Among the host factors described in the EORTC guidelines those found to be associated with the cases included prolonged use of corticosteroids, neutropenia, inherited severe immuno-deficiency and treatment with immunosuppressants other than steroids. It was noted that 17 of the 25 (68%) patients had underlying co-morbidities like uncontrolled diabetes mellitus 13 (52%), liver carcinoma (1), leukaemia (2) and HIV (1). Ours being a multispecialty tertiary care hospital, the underlying co-morbidities in patients were varied. There are very few reports on the performance of GM assay in patients with host factors other than haematologic malignancy. Uncontrolled diabetes, a well recognized risk factor for mucormycosis in India, is now being reported as a newly recognized risk factor for invasive aspergillosis other than tuberculosis and chronic obstructive pulmonary disease [14]. In our study diabetes was the predominant underlying condition with more than 50% of patients being diabetics [Table/Fig-1].
Of the 25 patients 10 required intensive care unit (ICU) management. Three of the 10 were in the ICU for less than 5 days one of whom expired and the other two improved. All the 7 patients who stayed in the ICU for 5-10 days improved clinically with antifungals which included voriconazole, posaconazole, Amphotericin B (liposomal and conventional) [Table/Fig-1]. A study analysing the performance of GA in a small group of mixed patient population in intensive care unit by Teering S et al., report the utility of GA to be quite low as they could not define a useful threshold based on their ROC analysis [15].
We did analyse concomitant administration of antibiotics and use of intravenous fluids containing plasmalyte which can raise the GM levels [10,11]. Since none of the patients included in this study were on these medications in the two weeks prior to sample collection for GA it was concluded that the raised GM levels could be attributed to invasive aspergillosis. False positivity due to other fungi was ruled out as none of the samples received from these patients yielded fungi other than Aspergillus.
Only 22 of the 25 patients were treated with antifungals. The empiric antifungal therapy was initiated based on clinical and radiological findings. Nine were on azoles (7 Voriconazole and 2 Posaconazole) and 10 of them on Amphotericin B and 3 were on both. 21 of the 22 treated patients improved clinically at the time of discharge but one of them expired and among the 3 untreated patients 2 were discharged against advice and one expired. In all 2 of the 25 patients expired during the course of their hospital stay [Table/Fig-1].
Salient features of this study are as follows. The prevalence of IPA was predominant in the age group of 31-50 years. The mean GM index value in healthy controls was 0.46 which is below the kit recommended cut-off of 0.5. Some of the risk factors especially diabetes mellitus hitherto not described a classical risk factor was found to be associated in more than 50% of our patients. Even with a small sample size of 25 patients with multiple associated co-morbidities, the performance of GM assay was found to be useful in the diagnosis of IPA and categorizing the possible group to probable based on the assay results.
Limitation
The small sample size is a definite limitation. The other factors such as performance of the test on stored samples and correlation of clinical factors using x-ray and not the recommended CT findings would have a bearing on the results and interpretation of the test.
Conclusion
Invasive aspergillosis is emerging as a significant problem in India especially among uncontrolled diabetic patients. These patients are most often managed in tertiary care multispecialty hospitals. The GM assay found to be useful for diagnosis of IPA among patients associated with factors described in EORTC guidelines could be a useful tool for early diagnosis among patients with other co-morbid conditions. Studies must be done on a larger population of patients with multiple co-morbidities to establish this. In this study, although we did establish an in-house cut-off for GM assay using both patient as well as control group, the results did not vary much and in the present context using the kit cut-off could still be recommended especially to compare data with other studies.