Laparoscopic Cholecystectomy (LC) is the treatment of choice in treating gallbladder disease substituting the conventional Open method of Cholecystectomy (OC) [1]. LC has improved surgical outcome in terms of reduced pain, morbidity and duration of convalescence compared to open cholecystectomy [2] but it is not a pain-free procedure, as many LC patients refrain from early recovery to normal activity which imperils the feasibility of LC as a low morbidity procedure [3].
There are various modalities available for postoperative pain relief ranging from parenteral analgesia (NSAIDS and opioids), epidural analgesia, peripheral nerve blocks, incisional infiltration and intraperitoneal instillation using local anaesthetics [4,5]. Prevention of transmission of nerve signals from the trauma site to the spinal cord and reduction of neurogenic local inflammation at the trauma site has been reported with the use of local anaesthetics. As large volumes are required in these techniques, ropivacaine, a newer amide, may be preferred due to less risk of cardiovascular toxicity and central nervous system side effects [1].
Hence, the present study was conducted to evaluate objectively, the efficacy of intraperitoneal instillation and rectus sheath block using ropivacaine for postoperative pain relief after laparoscopic cholecystectomy and to assess the supremacy of either of these techniques over administration of tramadol ‘on demand’- a conventional technique used in our institution.
Materials and Methods
After taking approval from the institutional ethical committee and informed consent from the patients, a prospective, single blind, randomized, controlled trial was conducted on 75 ASA grade I-III patients, aged >18 years scheduled to undergo laparoscopic cholecystectomy for gall stone disease under general anaesthesia over 6 months from January 2013 to June, 2013.
Patient Selection
Exclusion criteria were acute cholecystitis, history of allergy to local anaesthetics and inability to understand and use Visual Analog Scale (VAS), conversion to open cholecystectomy due to any reason and associated cardio-respiratory illness.
Patients were randomly assigned using opaque sealed envelope technique into 3 groups of 25 patients each depending on analgesic technique used: Group C (control) who received only rescue analgesic on pain, Group I (Intraperitoneal instillation) received intraperitoneal instillation of 0.25% ropivacaine 50ml and Group R (Rectus sheath block) received bilateral RSB with 30ml of 0.25% ropivacaine (15ml on each side).
Anaesthesia Technique
In the operation theater, after inserting an 18 G cannula in the right forearm, ringer lactate infusion was started. Non Invasive Blood Pressure (NIBP), peripheral oxygen saturation (SpO2) and ECG were monitored. Patients were pre-medicated with intravenous glycopyrrolate 0.2 mg, ondansetron 4 mg and tramadol 100 mg followed by induction with thiopentone 5mg/kg, intubation was facilitated with succinyl choline 1-1.5mg/kg with appropriate sized cuffed single use endotracheal tube. Anaesthesia was maintained with isoflurane 0.6-1%, oxygen and atracurium 25mg bolus followed by intermittent boluses of 5mg. At the end of surgery, neuromuscular blockade was antagonized with neostigmine 50μg/kg and glycopyrrolate 10μg/kg.
Technique of Intraperitoneal Instillation
In all the patients, access to peritoneal cavity was established through an umbilical incision. Pneumoperitoneum was created by insufflating CO2 using Verres needle (at 12-14 mmHg pressure) with patients in 20° Trendelenburg position. Immediately after creation of pneumoperitoneum and placement of first two trocars, suction port was inserted through 2nd trocar (epigastrium) under direct laparoscopic control, 0.25% ropivacaine 50ml was instilled over the upper surface of right and left lobes of liver and over gall bladder bed. All patients were maintained in 15-20° Trendelenburg position for about 2 minutes after instilling the solution.
Technique of Rectus Sheath Block
After induction, before start of the surgery rectus sheath block was administered on either side of the midline. A 22- gauge short bevel needle was inserted at point 4 finger breadths above and 4 finger breadth lateral to the umbilicus close to the lateral edge of the rectus abdominis muscle. The needle was advanced through the rectus abdominis muscle till a definitive resistance was felt as the needle was rolled over the posterior rectus sheath. Ropivacaine 0.25% (15ml) was deposited in the space between the rectus abdominis muscle and the posterior rectus sheath after a negative aspiration test. A similar block was repeated on the contralateral side of the abdomen.
Control group was not administered any analgesic other than the rescue analgesic.
Data Recording
Demographic data like age, sex and weight of the patients were recorded. Haemodynamic parameters like Heart Rate (HOUR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP) and Peripheral Oxygen Saturation (SpO2) were recorded as baseline (before induction) and after induction at 5, 10, 15 min and then every 15 min thereafter, till extubation. Surgical data like duration of surgery from skin incision to last suture and duration of pneumoperitoneum were also recorded.
During the pre-anaesthetic evaluation patients were given instructions for use of a 10 cm vertical VAS for measurement of pain and informed that they can request for rescue analgesia if required. The degree of spontaneous postoperative abdominal pain and shoulder pain were assessed using Visual Analog Scale (VAS) {0-no pain, 10- maximal pain} and Prince Henry Hospital Pain Score [10] (PHHPS) at 2h, 4 h, 6 h, 12 h, 24 h and 48 hours after surgery [Table/Fig-1].
Prince Henry Hospital Pain Score (PHHPS)

In Prince Henry Hospital Pain Score (PHHPS); Pain scoring was done with patient resting comfortably to assess the effect of analgesia on deep breathing and coughing.
Tramadol 50 mg i.v. was given as rescue analgesic for shoulder and abdominal pain when VAS>3 or PHHPS=3. Rescue analgesic requirement in terms of number of doses and total dose in mg as well as episodes of nausea and vomiting in 48 hour were recorded. Rescue antiemetic ondansetron 4mg i.v. was given as and when required and the total dose was recorded.
Patient satisfaction scores in postoperative period at 48 hour were recorded using ‘Likert scale’. Patients were asked to answer the question, ‘How would you rate your experience with analgesia you have received in postoperative period after surgery?’ using a 7-point Likert verbal rating scale [11]. Score of 5-7 was graded as acceptable satisfaction score.
Statistical Analysis
Data was entered and analysed with the help of MS Excel, EPI info 6 and SPSS 15.0. Power and sample size analysis showed that we need to take minimum of 25 patients in each group to detect statistically significant differences between two groups by keeping α= 0.05 and power of study 95%. The observations were compared statistically using student t-test/chi-square test/ANOVA. The p<0.05 was considered as statistically significant.
Results
All the three groups were comparable regarding demographic parameters [Table/Fig-2] and baseline hemodynamic parameters like HOUR, SBP, DBP and SpO2 (p>0.05). There was no significant intergroup variations in Heart Rate (HOUR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) and SpO2 in all the three groups at different time intervals intraoperatively (p>0.05) along with no significant change from the baseline.
Comparison of demographic variables in the three groups.
| Variable | Group C(n=25) | Group I(n=25) | Group R(n=25) | p-value |
|---|
| Age (y) | 46.04±13.68 | 47.64±14.76 | 43.64±11.79 | 0.594 (NS) |
| Weight (kg) | 57.80 ± 9.25 | 57 ± 9.01 | 58.6 ± 9.26 | 0.434 (NS) |
| Sex | Male | 5(20%) | 6(24%) | 10(40%) | 0.249 (NS) |
| Female | 20(80%) | 19(76%) | 15(60%) |
| ASA Grade | I | 21(84%) | 20(80%) | 18(72%) | 0.573(NS) |
| II | 4(16%) | 5(20%) | 7(28%) |
| Co-morbid conditions | Yes | 21(84%) | 20(80%) | 18(72%) | 0.573(NS) |
| No | 4(16%) | 5(20%) | 7(28%) |
| Duration of surgery (min) | 88.0 ±11.9 | 84.2 ±9.96 | 82.75± 8.34 | 0.088 (NS) |
| Duration of pneumoperitoneum (min) | 79.2 ±13.28 | 74.8 ±10.15 | 73.5±8.12 | 0.116 (NS) |
| Duration of anaesthesia (min) | 97.2 ±11.82 | 93.4 ±10.58 | 95.75±6.934 | 0.282 (NS) |
NS- not significant. Data are Mean ± SD or n (%)
Postoperative Analgesia
Postoperative abdominal pain as shown by mean VAS score which remained <4 and PHHPS which remained <3 throughout the study period of 48 hours indicated good postoperative pain control in all three groups.
Mean VAS scores for abdominal pain showed a significant difference among the three groups during first 6 hours. At 2 hour postoperatively, mean VAS score was significantly higher in Group C as compared to Group I and Group R while it was comparable between Group I and Group R (Group C > Group I ≈ Group R). At 4 hour postoperatively, mean VAS score was significantly higher in Group C as compared to Group I and Group R as well as in Group I as compared to Group R. {Group C > Group I > Group R}. At 6 hour postoperatively, VAS score was significantly less in Group R as compared to Group C and Group I while there was no statistical difference in VAS scores between Group C and Group I {Group C ≈ Group I > Group R}. After 6 hours, till 48 hours postoperatively, there was no significant difference in the VAS scores among the three groups {Group C ≈ Group I ≈ Group R}[Table/Fig-3].
Comparison of postoperative abdominal pain as assessed by Visual Analog Scale (VAS)
| Time | VAS | Group C(n=25) | Group I(n=25) | Group R(n=25) | p-value |
|---|
| Group C v/s Group I | Group C v/s Group R | Group I v/s Group R |
|---|
| 2hour | Mean±SDRange | 3.72± 1.131-6 | 1.12±0.7260-2 | 0.5±0.680-2 | p<0.001 | p<0.001 | 0.070 |
| 4hour | Mean±SDRange | 2.60±1.111-5 | 1.60±0.5001-2 | 0.8±0.830-2 | p<0.001 | p<0.001 | 0.011 |
| 6 hour | Mean±SDRange | 2.52±1.001-5 | 3.20±1.4432-7 | 1.1±0.850-2 | 0.091 | p<0.001 | p<0.001 |
| 12 hour | Mean±SDRange | 3.20±1.681-7 | 2.84±0.8981-5 | 2.75±1.251-5 | 0.597 | 0.529 | 0.994 |
| 24 hour | Mean±SDRange | 3.76±1.532-8 | 3.60±1.1182-6 | 3.74±1.412-6 | 0.910 | 0.956 | 0.520 |
| 48 hour | Mean±SDRange | 2.64±1.111-5 | 3.28±1.1371-5 | 3.1±1.291-5 | 0.133 | 0.169 | 0.992 |
Mean PHHPS score for abdominal pain showed significant difference among the three groups during first 12 hours. At 2 hour and 4 hour postoperatively, mean PHHPS score was significantly higher in Group C as compared to Group I and Group R while there was no significant difference between Group I and Group R. {Group C > Group I ≈ Group R}. At 6 hour and 12 hour postoperatively, the mean PHHPS score was significantly less in Group R as compared to Group C and Group I whereas it was comparable in Group C and Group I. {Group C ≈ Group I > Group R}. After 12 hours, till 48 hours postoperatively, there was no significant difference in the mean PHHPS score among the three groups. {Group C ≈ Group I ≈ Group R} [Table/Fig-4].
Postoperative abdominal pain as assessed by Prince Henry Hospital Pain Score (PHHPS)
| Time | PHHPS | Group C (n=25) | Group I (n=25) | Group R (n=25) | p-value |
|---|
| Group C v/s Group I | Group C v/s Group R | Group I v/s Group R |
|---|
| 2 hour | Mean±SDRange | 1.6±1.20-3 | 0.4±0.50-1 | 0.0±0.00-0 | p<0.001 | p<0.001 | 0.207 |
| 4 hour | Mean±SDRange | 1.36±0.990-3 | 0.56±0.510-1 | 0.1±0.310-1 | p<0.001 | p<0.001 | 0.060 |
| 6 hour | Mean±SDRange | 1.4±0.90-3 | 1.8±0.91-3 | 0.7±0.70-2 | 0.149 | 0.008 | p<0.001 |
| 12 hour | Mean±SDRange | 1.9±0.90-3 | 1.9±0.71-3 | 1.2±0.90-2 | 1.000 | 0.008 | 0.008 |
| 24 hour | Mean±SDRange | 2.3±0.71-3 | 2.3±0.71-3 | 2.2±0.71-3 | 1.000 | 0.706 | 0.706 |
| 48 hour | Mean±SDRange | 1.9±0.71-3 | 2±0.71-3 | 1.7±0.71-3 | 0.709 | 0.709 | 0.258 |
None of the patients experienced significant shoulder pain (i.e., VAS>3, PHHPS=3) at any time during the study period and all the three groups were statistically comparable regarding postoperative shoulder pain (p >0.05) as depicted by VAS and PHHPS scores.
Rescue Analgesic Requirement
All the patients in the three groups required rescue analgesic during postoperative period of 48 hours. The first dose of rescue analgesic was required significantly earlier in Group C (1.72 ± 0.67 hour) as compared to Group I (7.84 ± 1.34 hour), p<0.001 and Group R (16.16 ±4.73 hour), p<0.001, the difference was also significant between Group R and Group I, p<0.001. In initial 6 hours postoperatively, all patients (100%) of Group C required rescue analgesic, while only 4 (16%) patients in Group I, and no patient (0%) in Group R demanded for rescue analgesic. Total number of doses of rescue analgesic required in first 6 hours were significantly more in Group C (28) as compared to Group I (4) and Group R(0), p<0.001; and it was significantly lower in Group R (0) as compared to Group I (4), p=0.043. Between 6-24 hours postoperatively, all the patients in the three groups required rescue analgesic. In next 24 hours, all the patients in all the groups required rescue analgesic and this requirement was comparable in terms of number of doses and total dose (p>0.05). For maintenance of postoperative analgesia patients of Group R required 1-4 doses, Group I required 3-5 doses and Group C required 5-7 doses of tramadol in 48 hours [Table/Fig-5].
Distribution of patients according to total number of doses in 48 hrs.
*Average number of rescue analgesic dose required by each patient
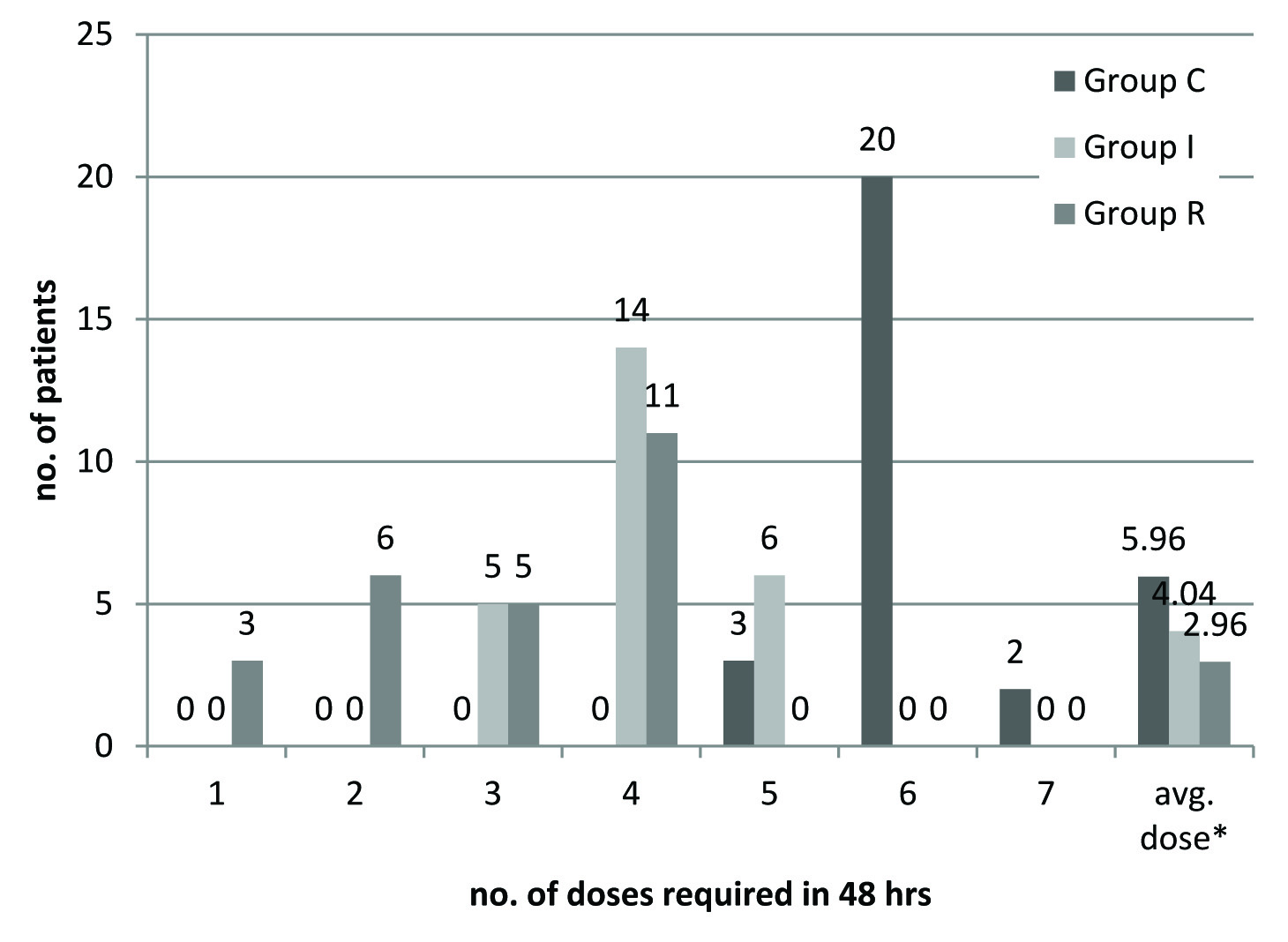
The mean number of doses of rescue analgesic required by each patient over 48 hours postoperatively in Group C (5.96 ±0.45) was significantly higher than Group I (4.04 ±0.67), p<0.001 and Group R (2.96 ±1.09), p<0.001 as well as in Group I (4.04 ±0.67) as compared to Group R(2.96 ±1.09), p<0.001 [Table/Fig-6].
Comparison of requirement for rescue analgesic in postoperative period.
| | Group C(n=25) | Group I(n=25) | Group R(n=25) | p-value |
|---|
| Group C v/sGroup I | Group C v/sGroup R | Group I v/sGroup R |
|---|
| Time for 1st dose (h) | 1.72 ± 0.67 | 7.84 ± 1.34 | 16.16 ±4.73 | <0.001 | <0.001 | <0.001 |
| 0-6 hour | No. of pt requiring rescue analgesic | 25 (100%) | 4(16%) | 0(0%) | <0.001 | <0.001 | 0.043 |
| No. of patients requiring | Single dose | 22 | 4 | 0 | | | |
| two doses | 3 | 0 | 0 |
| Total no. of doses | 28 | 4 | 0 | p<0.001 | p<0.001 | 0.043 |
| Total dose (in mg) | 1400 | 200 | 0 | | | |
| 6-24 hour | No. of pt requiring rescue analgesic | 25 (100%) | 25 (100%) | 25 (100%) | | | |
| No. of patients requiring | single dose | 0 | 2 | 8 | | | |
| two doses | 6 | 18 | 14 |
| three doses | 19 | 5 | 0 |
| Total no of doses | 69 | 53 | 36 | 0.143 | 0.002 | 0.068 |
| Total dose (in mg) | 3450 | 2650 | 1800 | | | |
| 24-48 hour | No. of pt requiring rescue analgesic | 25 (100%) | 25 (100%) | 25 (100%) | | | |
| No. of patients requiring | single dose | 0 | 6 | 10 | | | |
| two doses | 23 | 19 | 14 |
| three doses | 2 | 0 | 0 |
| Total no of doses | 52 | 44 | 38 | 0.420 | 0.136 | 0.515 |
| Total dose (in mg) | 2600 | 2200 | 1900 | | | |
| 0-48 hour | No. of pt requiring rescue analgesic | 25 (100%) | 25 (100%) | 25 (100%) | | | |
| Total no. of dose required by each patient (Mean ±SD) | 5.96 ±0.45 | 4.04 ±0.67 | 2.96 ±1.09 | <0.001 | <0.001 | <0.001 |
| Total dose (in mg) by each pt (Mean ±SD) | 298 ±22.73 | 202.0±33.78 | 148.0±54.92 | <0.001 | <0.001 | <0.001 |
Data are Mean ± SD or n (%)
Thus, rescue analgesic consumption was reduced by 50.33% with RSB and 32.21% by intraperitoneal instillation as compared to control group.
Emetic episodes were minimal in this study. Only two patients, 1 (4%) each, in Group C and Group I had retching and were given ondansetron. Thus, anti-emetic requirement and emetic episodes were found to be comparable in all the three groups (p>0.05).
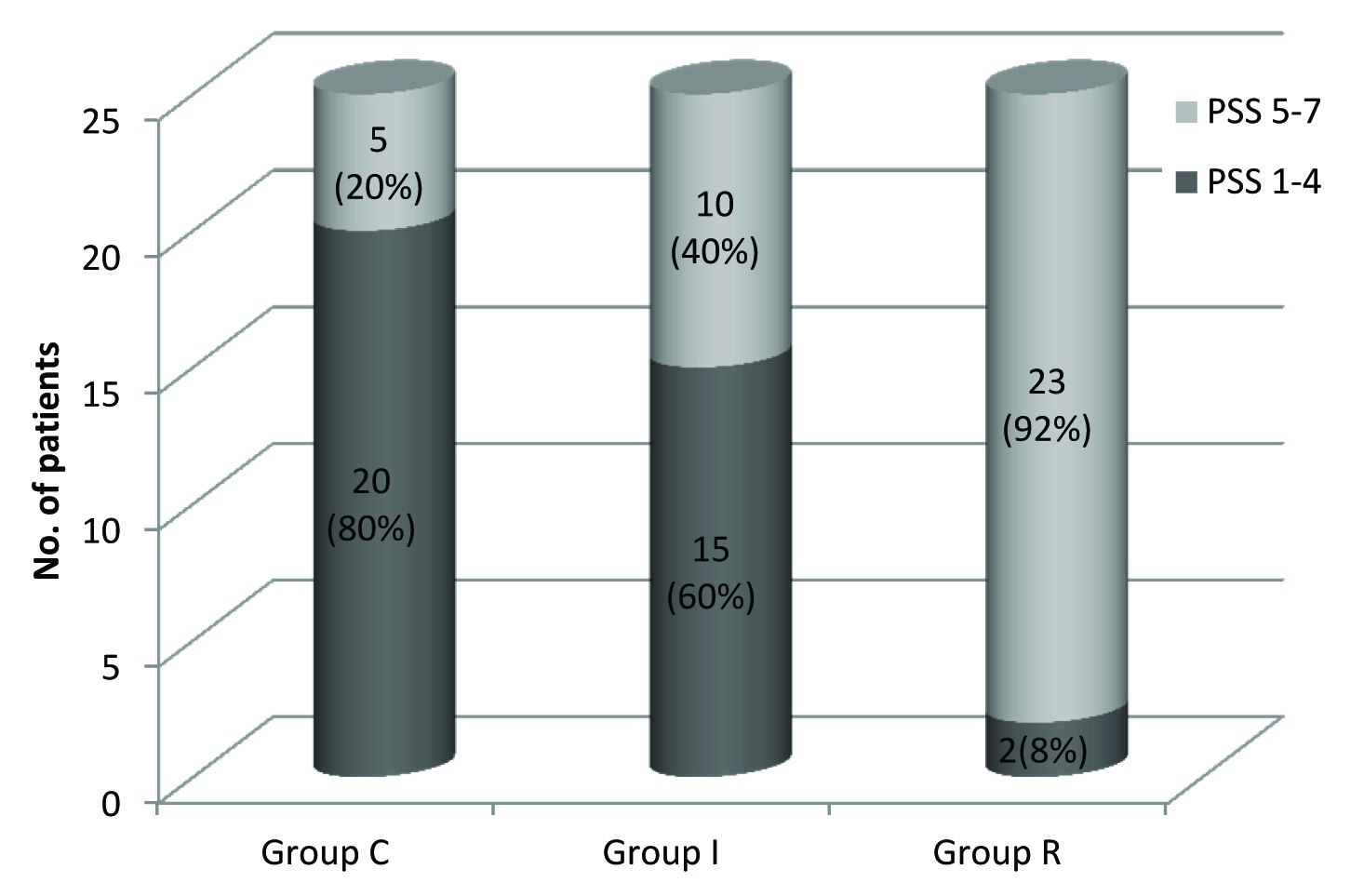
There was significant difference in the patient satisfaction score among the three groups. In Group R, 23(92%) patients had acceptable PSS (i.e., 5-7), whereas in Group I 10(40%) patients, and in Group C, only 5(20%) patients had acceptable PSS, Group R > Group I > Group C. (p=0.000) [Table/Fig-7].
Comparison of patient satisfaction scores among the three groups
Discussion
Pain after LC is comprised of three different components: incisional pain (somatic pain), visceral pain, and shoulder pain (referred visceral pain) [12]. Pain after LC demonstrates a high inter-individual variability in intensity as well as duration and is also largely unpredictable. Intensity of pain is highest on the day of surgery and on following day, subsequently declining to low levels within 3–4 days [5]. Moreover, it has been hypothesized that intense acute pain which occurs after LC may also lead to establishment of chronic pain (e.g., post LC syndrome) [13].
In previous studies, ropivacaine has shown less cardiotoxicity and central nervous system side effects, compared to bupivacaine in same plasma concentration even in large dose (300 mg) of intraperitoneal instillation [8,14,15]. In this study also 0.25% ropivacaine was used in large volumes for intraperitoneal instillation (50ml; 125mg) and RSB (30ml; 75mg) without any toxic side effects. In the present study, both techniques were used as pre-emptive analgesia which prevents the formation of central sensitization to painful stimuli by decreasing response from pain sensation [3].
The outcomes of this study demonstrate efficacy of the intraperitoneal instillation of ropivacaine and rectus sheath block in reducing postoperative pain significantly after LC as shown by lower pain scores (VAS and PHHPS), delay in requirement of first dose of rescue analgesic and reduction in total rescue analgesic consumption.
Intraperitoneal instillation has been evaluated previously for providing postoperative analgesia after laparoscopic cholecystectomy using ropivacaine [1], bupivacaine [16], levobupivacaine [17] and lignocaine with positive outcomes. Local anaesthetics act on visceral nociceptors of peritoneum. Another possible mechanism is absorption from the large peritoneal surface [15].
However, a negative outcome to intraperitoneal instillation [18] could be attributed to the administration of local anaesthetic intraperitoneally at the end of surgery rather than preemptively. They observed a decrease in rescue analgesic consumption, but this could not reach statistical significance.
Many factors have been identified that may influence the benefits of intraperitoneal anaesthesia like dose and concentration of local anaesthetic used, site of instillation (subdiaphragmatic versus subhepatic), timing of instillation (before and after surgery), pneumoperitoneum (volume, pressure and temperature), volume of residual CO2 (causing diaphragmatic irritation), spillage of bile and blood (may interfere with absorption), degree of non-visceral pain (e.g., from incision site), instillation in head down position versus supine [14].
In the present study, intraperitoneal instillation was done before surgery, using 0.25% ropivacaine in head down position and pressure of pneumoperitoneum was limited to 12mmHg. All these factors may have contributed to reduction in postoperative pain after intra-peritoneal instillation observed in our study.
RSB has been used to achieve postoperative analgesia in a variety of clinical settings including umbilical hernia repair [6,7,19], abdominoplasty [20], following laparoscopy [21,22], upper abdominal [23] and major gynaecological surgery [24]. There are wide discrepancies in the results from various studies due to different age group of the patients studied, operator expertise, extent and nature of surgery and technique of RSB.
RSB aims to bathe the nerves of the rectus sheath plexus in local anaesthesia as they pass through the rectus sheath space between the rectus abdominis muscle and the posterior layer of the sheath [24]. Local anaesthetic deposition within the posterior rectus sheath bilaterally provides dense analgesia over the middle anterior wall from the xiphoid process to symphysis pubis [25]. The RSB is a field block which covers multiple nerves (branches of T9, T10 and T11 intercostal nerves) to provide nearly complete analgesia. It is possible that a single injection may not cover all the nerve segments [19].
A significant advantage of RSB technique is early mobility. Excellent analgesia along with minimal motor block of the limbs and no mandatory connection to infusion devices allow early patient mobilisation. This leads to major benefits including reduced potential for deep vein thrombosis and pulmonary embolus, lesser incidence of atelectasis and respiratory infection, and minimal motor deconditioning [25]. In present study, significantly reduced pain scores (PHHPS) on coughing and movement were observed in RSB group upto 12 hours as compared to intraperitoneal group and control group.
Many variations in the technique of administration of this block have superseded the traditional blinded technique used by anaesthetists before the advent of ultrasound guided nerve blocks. A more contemporary approach to the RSB involves the use of ultrasound and block under direct vision during surgery [24]. USG guided blocks though safer and more accurate [7,26], require more expertise and specialised equipment.
Shoulder tip pain after LC has been attributed to trapping of carbon dioxide gas beneath the right haemidiaphragm after deflation of the abdomen. Chundrigar et al., stated that when abdomen is deflated through epigastric cannula, the tip of which was placed above the right lobe of liver to allow all gas in this region to escape, it results in lower incidence of shoulder pain [27]. Similar technique was followed in this study. This could be the reason that none of the patients in present study complained of shoulder tip pain.
A decrease in forced vital capacity and tendency to hypoxemic episodes (SaO2<92%) was reported in patients receiving intraperitoneal bupivacaine as compared to control group in the first few hours after surgery which was attributed to partial paresis of phrenic nerve due to local anaesthetic blockade [15]. Traditional RSB relies on anatomical landmarks and loss of resistance; there is also the remote potential for perforation of intraperitoneal structures and epigastric blood vessels [28]. However, such technique related complications were not encountered in our study.
Limitation
The present study has certain limitations. Firstly, RSB was performed using traditional blind technique in this study. Therefore, the accuracy of placement of block cannot be judged and all the cases which were given RSB were considered as successful block for assessment of postoperative analgesia. Use of ultrasound guidance could have reduced the cases of failed block which were not recognized. Secondly, administration of RSB requires additional time and expertise of the performer whereas intraperitoneal instillation can be done by the surgeon himself. Assessment of the time for administration of block and surgeon satisfaction scores, could have further led credence to this technique. Inspite of above limitations certain conclusions can be drawn from the study.
Conclusion
Thus, we conclude that pre-emptive administration of rectus sheath block or intraperitoneal instillation of 0.25% ropivacaine provided better postoperative analgesia as compared to control group after laparoscopic cholecystectomy. Among these two techniques, rectus sheath block seemed to be superior in providing postoperative analgesia. Hence, this study favours the administration of rectus sheath block pre-emptively for postoperative pain relief after laparoscopic cholecystectomy.
NS- not significant. Data are Mean ± SD or n (%)