Introduction
Peptic ulcer is a common disease that affects millions of people worldwide. Considering its global prevalence finding new approach for treating is important.
Aim
The aim of this study was to investigate the effect of zinc sulfate on gastric and duodenal ulcer treatment.
Materials and Methods
This double-blind clinical trial study was done on 90 patients who were admitted to the gastrointestinal endoscopy clinic of Tohid hospital in Sanandaj, Iran. All patients were diagnosed with gastric and duodenal ulcers. They were randomly divided into two-intervention and control groups, using block randomization with block sizes of 4. Patients and researcher were unaware of the grouping. To assess the level of zinc, blood samples were taken. In case of positive Rapid Urease Test (RUT), triple therapy regimen including amoxicillin, clarithromycin and omeprazole was administered for two weeks. For intervention group in addition to "triple therapy", an oral dose of Zinc Sulfate 220mg capsules were administered daily, while the control group received placebo capsules.
Results
A total of 54.5% and 57% of the patients in the intervention and control groups had gastric ulcer respectively. The Rapid Urease Test (RUT) result of 72.7% of intervention group and 83.3% of control group was positive (p = 0.24). Serum zinc level of 20.9% of intervention group and 35.7% of control group was lower than the normal level (p = 0.13). The mean of serum zinc level of intervention group and control group were 81.9 and 78.9 mg dL respectively (p = 0.4). After intervention, peptic ulcer in 81.8% of the intervention group and 83.3% of the control groups were improved (p= 0.85). Response to treatment were higher in patients with normal zinc levels compared to patients with abnormal levels (77.5% vs. 22.5%, p=0.019).
Conclusion
A daily dose of 220mg zinc sulfate was not significantly effective on peptic ulcer. However, patients with normal zinc levels had better ulcer treatment.
Introduction
Peptic ulcer has plagued mankind for centuries. The first suggested description of gastric hemorrhage attributed to an ulcer is in the Egyptian Papyrus Ebers [1]. Gastric and duodenal ulcers significantly affect millions of people worldwide [2]. Of adult US residents, 10% reported having physician-diagnosed ulcer disease, and one third of these individuals reported having an ulcer in the past year. The disease is responsible for 15,000 deaths annually. The annual direct and indirect costs of the disease are estimated to be more than $10 billion [3]. A meta-analysis has reported that 95% of all hospitalized ulcer cases in the USA were attributable to H pylori infection, main risk factors are-use of non-steroidal anti-inflammatory drugs (NSAIDs), minor tranquillisers and tobacco smoking [4,5]. Over the past few decades, dietary factors are taken into consideration as effective factors on digestive diseases including peptic ulcer [6].
Zinc is an essential mineral for optimal development of human body. It is ubiquitous within cells in contrast to iron, which is contained in defined cellular components and has defined physiological roles. The role of zinc in biology can be grouped into three general functional classes, namely catalytic, structural and regulatory functions [7]. Furthermore, zinc is an important metabolic requirement for growth and repair of squamous tissue [8]. Numerous aspects of cellular metabolism are dependent on zinc. It is required for the function of approximately 100 enzymes and it plays a role in immune function, protein synthesis, wound healing, DNA synthesis and cell division [7–10].
Effect of zinc on peptic ulcer treatment has been described in animal studies. Moreover, other studies have shown the protective action of zinc ions on the gastric mucosa [9,10]. Frommer [11] showed zinc effectiveness on gastric ulcers, but Yazdanpanah et al., in their study found no significant association between zinc supplementation and peptic ulcer treatment [12]. They recommended further studies with higher doses of zinc to determine effective dose. The aim of this study was to investigate the effect of zinc sulfate on gastric and duodenal ulcer treatment.
Materials and Methods
This randomized, double-blind clinical trial study was done on 90 patients who referred to the Digestive Endoscopy Center of Sanandaj Tohid Hospital from 2013 to 2014. Inclusion criteria were peptic ulcer during endoscopy and age above 20 years. Exclusion criteria were clear symptoms indicating malignancy, malignancy in pathology, patient reluctance to endoscopy, unwillingness in continuing medication, discontinuation of the medication for more than two days and diseases that impair absorption (cirrhosis, celiac disease).
Endoscopy was performed for patients by subspecialist of gastroenterology using Olympus 160 Video Endoscope Endoscopy System. Then patients with gastric and duodenal ulcers were enrolled in the study. After explaining the procedure, written informed consent was taken from participants.
Total of 90 patients were divided in two-intervention and control groups using the randomized block method. Based on study variables clinical interview was performed for each patient and data were documented in questionnaires. Blood samples were taken and sent to the laboratory to measure the level of zinc. Standard treatment was started for both groups according to the results of RUT. Triple therapy regimen including amoxicillin, clarithromycin, and omeprazole was administered for two weeks if the RUT test was positive. Based on ulcer size, triple therapy regimen was prescribed for 2 weeks followed by 4 weeks of omeprazole for 3 cm ulcers. If the ulcer was less than 3 cm triple therapy regimen was prescribed for 2 weeks followed by 2 weeks of omeprazole. In the case of negative RUT test, treatment was performed only with omeprazole.
H pylori AB-I was checked in false negative RUT cases such as in patients with gastrointestinal bleeding, consumption of Proton Pump Inhibitors (PPI), or in history of antibiotic use in previous 2 weeks. Depending on the positive or negative RUT, patients were treated as RUT positive or RUT negative respectively. In addition to triple therapy, a daily dose of zinc sulfate 220mg (Alhavi Pharmaceutical Co.) were prescribed in the intervention group orally. In the control group only placebo was given to the patients. After intervention the ulcers were assessed by second endoscopy performed by the same physician and their size were documented.
From 90 cases who were participated in the study 4 patients were excluded from the study (2 patients underwent surgery due to bleeding and 2 of them had no desire to continue with the study) [Table/Fig-1].
Flow diagram of the progress through the phases of a two-group parallel randomized trial.
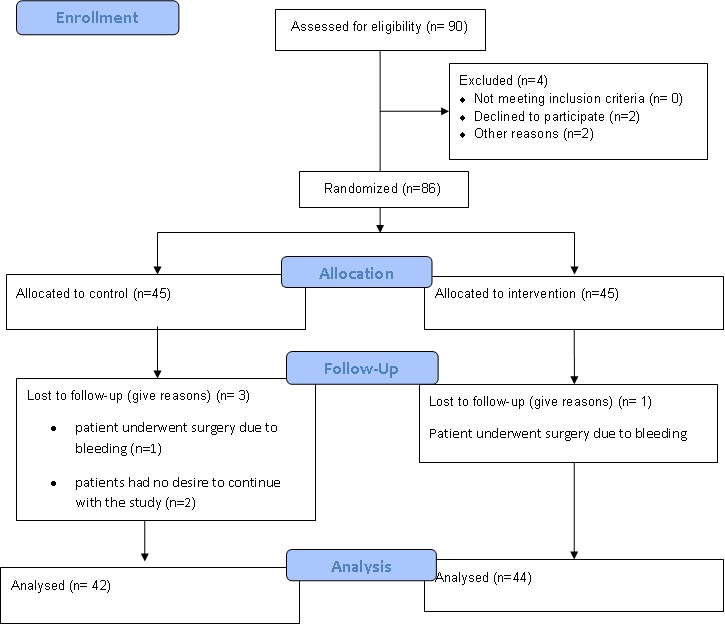
Statistical Analysis
Data were analysed by Statistical Package for Social Sciences (SPSS) software version 18.0. To compare the improvement of peptic ulcer, descriptive statistics, chi-square, and fisher exact test were used. T-test was used for quantitative variables such as age, ulcer size and level of zinc in both intervention and control groups.
This study has been approved by the ethics committee of Kurdistan University of Medical Sciences and also has been registered in Iranian Registry for Clinical Trials with code no. IRCT2014062212789N4.
Results
Findings of this study showed that, male population in the intervention and control group was 81.8% and 81% respectively. Mean age of the patients in the intervention and control group was 47.5 ± 17.2 years and 52.6 ± 18.4 years, respectively (p=0.02). In both groups, use of NSAIDs was the same (52.3 % vs. 54.8%).
In the intervention group 55.5% of the ulcers were located in the stomach and 45.5% of them were found in duodenum. However, in the control group, 59.6% of the ulcers were located in the stomach and 40.4% of them were found in the duodenum (p>0.05). The result of RUT and IgG was similar in both groups. RUT was positive in 72.7% of the intervention and 83.3% of the control group (p= 0.24) [Table/Fig-2].
Comparison of two groups in terms of different variables.
| Variable | Group | Intervention | Control | p |
|---|
| No. (%) | No. (%) |
|---|
| Gender | Male | 36 (81.8) | 34 (81.0) | 0.92 |
| Female | 8 (18.2) | 8 (19.0) |
| Smoking | Yes | 19 (43.2) | 11 (26.2) | 0.1 |
| No | 25 (56.8) | 31 (73.8) |
| Alcohol | Yes | 3 (6.8) | 1 (2.4) | 0.62 Fisher |
| No | 41 (93.2) | 41 (97.6) |
| Use of Non-steroidal anti-inflammatory drugs | Yes | 23 (52.3) | 23 (54.8) | 0.82 |
| No | 21 (47.7) | 19 (45.2) |
| Ulcer site | Stomach | 24 (55.5) | 25 (59.6) | 0.64 |
| Duodenum | 20 (45.5) | 17 (40.4) |
| RUT | Positive | 32 (72.7) | 35 (83.3) | 0.24 |
| Negative | 12 (27.3) | 7 (16.7) |
| IgG | Positive | 32 (72.7) | 34 (81.0) | 0.45 |
| Negative | 12 (27.3) | 8 (19.0) |
There was no significant difference between both groups for epigastric pain, night pain, epigastric burning, nausea, vomiting, melena, and rectorrhagia (p>0.05). However, frequency of immediate pain after meal in the intervention and control groups was 54.5 % and 28.6% respectively (p=0.015). Pain with interval after meal, was 36.4% in the intervention group and 61.9% in the control group (p=0.018) was significant [Table/Fig-3].
Frequency of symptoms in the intervention and control groups.
| Variable | Group | Intervention | Control | p |
|---|
| No. | (%) | No. | (%) |
|---|
| Epigastric pain | Yes | 39 | 88.6 | 36 | 85.7 | 0.68 |
| No | 5 | 11.4 | 6 | 14.3 |
| Pain immediately after meal | Yes | 24 | 54.5 | 12 | 28.6 | 0.015 |
| No | 20 | 45.5 | 30 | 71.4 |
| Pain with interval after meal | Yes | 16 | 36.4 | 26 | 61.9 | 0.018 |
| No | 28 | 63.6 | 16 | 38.1 |
| Night pain | Yes | 26 | 59.1 | 22 | 52.4 | 0.53 |
| No | 18 | 40.9 | 20 | 47.6 |
| Stomach pain relieved by antacids | Yes | 24 | 54.5 | 17 | 40.5 | 0.19 |
| No | 20 | 45.5 | 25 | 59.5 |
| Epigastria burning | Yes | 24 | 54.5 | 23 | 54.8 | 0.98 |
| No | 20 | 45.5 | 19 | 45.2 |
| Nausea | Yes | 29 | 65.9 | 28 | 66.7 | 0.94 |
| No | 15 | 34.1 | 14 | 33.3 |
| Vomiting | Yes | 25 | 56.8 | 22 | 52.4 | 0.68 |
| No | 19 | 43.2 | 20 | 47.6 |
| Hematemesis | Yes | 14 | 42.9 | 18 | 42.9 | 0.29 |
| No | 30 | 57.1 | 24 | 57.1 |
| Melena | Yes | 30 | 45.5 | 23 | 54.8 | 0.39 |
| No | 14 | 54.5 | 19 | 45.2 |
| Rectorrhagia | Yes | 8 | 18.2 | 3 | 7.1 | 0.2 Fisher |
| No | 36 | 81.8 | 39 | 92.9 |
The mean of peptic ulcer size in the intervention and in the control groups were 11.1 ±3.6 and 15.4 ± 7.3 mm (p=0.0001). The mean of zinc level in the intervention group was 81.9±16.4 mg/dl also for 79.1% of them the level of zinc was normal. The mean of zinc level in the control group was 78.9 ± 17.4 mg/dl. For 64.3% of the patients in control group the level of zinc was normal (p=0.4) [Table/Fig-4].
Comparison of the level of zinc in the intervention (A) and control (B) groups.
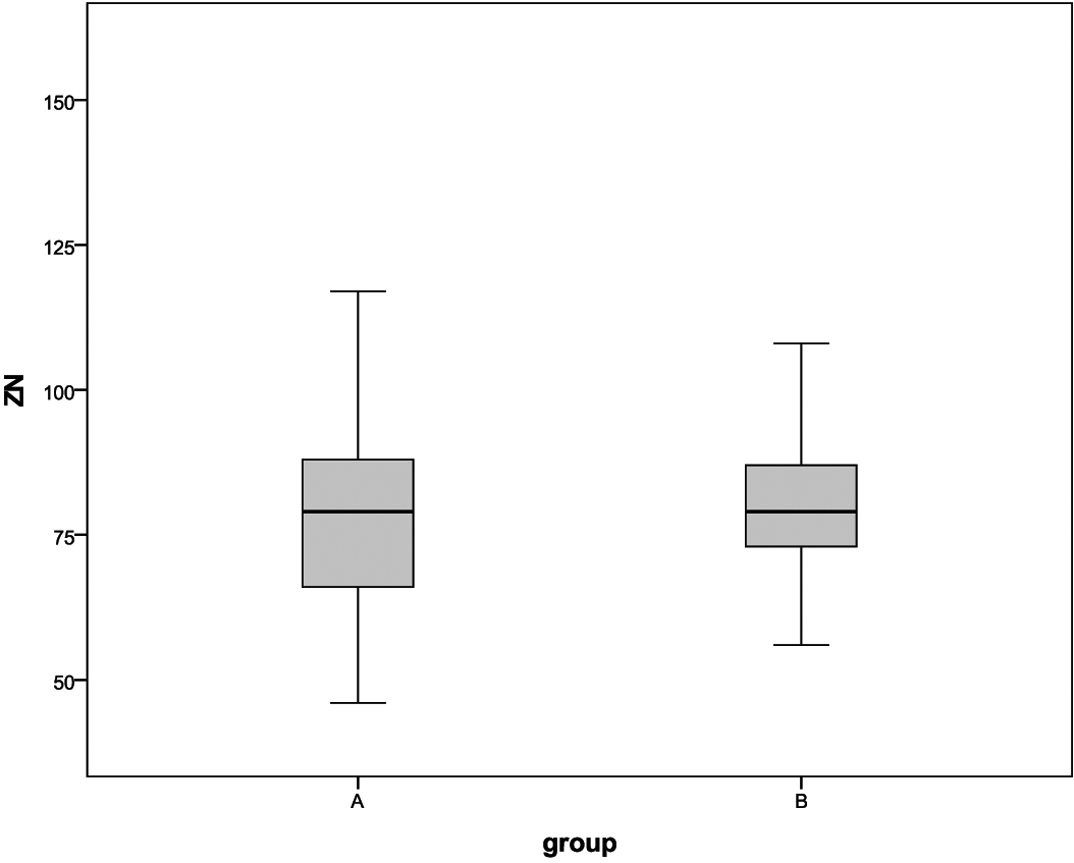
The response to treatment in the intervention and control groups did not show a significant difference statistically (p=0.85) [Table/Fig-5]. However, in intervention group positive response was 81.8% and patients with normal zinc serum levels had better ulcer treatment (77.5% Vs 22.5%, p=0.019) [Table/Fig-6].
Response to treatment in the two groups.
| Group→ | Intervention | Control | p |
|---|
| Response to Treatment ↓ | No. | (%) | No. | (%) |
|---|
| Positive | 36 | 81.8 | 35 | 83.3 | 0.85 |
| Negative | 8 | 18.2 | 7 | 16.7 |
Relationship between the results of treatment and zinc serum level in the two groups.
| Response to ↑Treatment/ | Yes | No | p |
|---|
| Zinc serum level ↓ | No. | (%) | No. | (%) |
|---|
| Lower than normal | 16 | 22.5 | 8 | 57.1 | 0.019 |
| Negative | 55 | 77.5 | 7 | 42.9 |
Discussion
Helicobacter pylori is the main cause of peptic ulcer disease. Physiologically essential elements; Fe, Zn, and Cu are of great clinical concern in medicine. Their deficiencies are among the important health problems [13]. The studies have recently been focused on the possible anti- Helicobacter pylori effects of dietary metals [14–17].
In our study the treatment response in the intervention and control groups were 81.8% and 83.3% respectively which showed no significant difference. The study results revealed that zinc sulfate had no statistically significant effect on peptic ulcer. In a study by Yazdanpanah et al., zinc sulfate administered every other day, after intervention, the ulcer size in the intervention group was less than the control group; however, the difference was not statistically significant [12]. In our study also the ulcer size in the intervention group was less than the control group and difference was statistically significant. It seems that zinc sulfate can reduce the ulcer size and for better result the more dose of zinc sulfate is required. Zinc sulfate o.d was given to the patients in the present study. In Frommer’s study zinc sulfate 220mg was given orally three times a day for three weeks in intervention group. The ulcer healing rate was three times that of control group. This difference was significant [11]. Also, Pories revealed that oral medication with zinc sulphate is safe at the level of 220mg. t.i.d. for periods up to 61 days and it accelerate wound healing as well as systemic therapy with zinc is beneficial during tissue repair in man just as it is in rats [18]. Rodrigues et al., conducted a study to demonstrate the protective action of zinc ions on the gastric mucosa of rats. The results showed that zinc had positive effect on the healing of gastric ulcer [10].
In our study, there was no difference between groups in terms of the serum zinc level, but the number of cases with lower level in the intervention group was less than the control group. In a study by Bandyopadhyay et al., a significant increased value (p<0.01) of zinc content in gastric mucosa of patients with peptic ulcer diathesis was noted. They concluded that the low serum zinc level of the peptic ulcer patients is possibly due to the positive shift for the zinc from serum to the gastric mucosa [19]. Plasma and gastroduodenal levels of zinc reflect the stage of the pathological process in peptic ulcer [20].
Previous studies have indicated the positive effect of zinc on ulcer healing in animal models [21,22]. In a study conducted by Garcia, a daily dose of 600mg zinc sulfate was more effective than Famotidine in the treatment of duodenal ulcers [23]. Findings of study by Troskot et al., indicated that zinc plays an important cytoprotective role in duodenal ulcer disease [24].
In the present study in terms of the epigastric pain, night pain, pain relief with antacids, epigastric burning, nausea, vomiting, haematemesis, melena and rectorrhagia there was no significant difference between the intervention and control groups. However, there was statistically significant difference between the two groups in terms of pain immediately after meal and pain with interval after meal. Frequency of pain immediately after meal was higher in the intervention group; whereas, pain with interval after meal was higher in the control group. Incidence of gastric ulcers (57%) was higher than duodenal ulcers; however, there was no statistical difference between the two groups in terms of location of the ulcer.
In a study conducted by Barazandeh et al., on North West’s population of Iran incidence of gastric ulcer was 3.3% and duodenal ulcer has been reported as 4.9% [25]. This rate was lower than the reported prevalence in previous studies in Asia which were based on endoscopy in the general population [26–28]. In a systematic review by Li et al., incidence of gastrointestinal ulcer, gastric ulcer and duodenal ulcer in china were 17.2%, 1.6%, and 13.3% respectively [29]. According to medical examination and assessment of hospitalized patients, annual global incidence of gastric ulcer was 0.1 -0.19% and 0.03 -0.17%, respectively [30]. Results of this study is similar to the results of epidemiologic studies conducted in Europe which showed a prevalence of 4.1% (2% gastric ulcer and 2.1% duodenal ulcer) [31] to 6.2% (gastric ulcer 2.3% and duodenal ulcers 3.9%) [32]. in all of these studies prevalence of gastric ulcers was less than duodenal ulcer. In our study, incidence of gastric ulcer was more than duodenal ulcer which could be explained by different statistical population. Another reason could be that other studies were conducted in the general population, while our study was conducted on patients with gastrointestinal symptoms referring for endoscopy.
Mean age of patients in this study was 47.5 and 52.6 years in the intervention and control groups respectively. In the studies conducted in Iran, mean age was between 40 and 53.3 year [33,34]. In other international studies mean age was between 48-57 years [35,36]. Studies which conducted in Iran showed that with promotion of public health, mean age increased.
RUT result for the detection of Helicobacter pylori were the same in both groups and was about 78%. In a study conducted on 200 patients, 82% of the patients were infected with Helicobacter pylori [37]. Many studies on biological microscopy in China revealed high prevalence of Helicobacter pylori [30]. In 73% to 100% of the patients with duodenal ulcer and 65% to 100% of the patients with gastric ulcers, high infection rate with Helicobacter pylori were reported [38,39]. In a 10-year study in Korea, by Jang et al., incidence of peptic ulcer and especially duodenal ulcer had decreasing trend [40]. Good management and promotion of community health in the west lead to the eradication of H. pylori followed by decrease in the rate of duodenal ulcer [41].
Limitation
+++The limitation of our study was that; some patients who were treated during the study did not follow-up.
Conclusion
Daily administration of 220mg of zinc sulfate reduced peptic ulcer size and also patients with normal levels of serum zinc had better healing processes. It seems that for better effect high dose of zinc sulfate is required.
[1]. Baron JH, The relationship between basal and maximum acid output in normal subjects and patients with duodenal ulcerClin Sci 1963 24:357-70. [Google Scholar]
[2]. Proceedings of the 2015 International Conference on Medicine and Biopharmaceuticals. China: World Scientific; 2015 [Google Scholar]
[3]. Sonnenberg A, Everhart J, The prevalence of self-reported peptic ulcer in the United StatesAm J Public Health 1996 86(2):200-05. [Google Scholar]
[4]. Kurata JH, Nogawa AN, Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori and smokingJ Clin Gastroenterol 1997 24:2-17. [Google Scholar]
[5]. Rosenstock S, Risk factors for peptic ulcer disease: a population based prospective cohort study comprising 2416 Danish adultsGut 2003 52(2):186-93. [Google Scholar]
[6]. Gu GG, Yong DG, Geng BQ, Anti-gastric ulcer activity of zinc sulfadiazine in ratsZhongguo Yao Li XueBao 1990 11(5):460-62. [Google Scholar]
[7]. Cousins RJ, Zinc. In: Filer LJ, Ziegler EE, editorsPresent Knowledge in Nutrition 1996 7th edWashington DCInternational Life Science Institute Nutrition Foundation:293-306. [Google Scholar]
[8]. Kadakia S, Wong R, Maydonovitch C, Nelson N, Henkin R, Serum and tissue zinc concentrations in patients with endoscopic esophagitisDigest Dis Sci 1992 37(4):513-16. [Google Scholar]
[9]. Shah D, Sachdev HP, Zinc deficiency in pregnancy and fetal outcomeNutr Rev 2006 64:15-30. [Google Scholar]
[10]. Rodrigues LE, Galle P, Siry P, Vinhaes A, The protective effect of zinc on gastric ulceration caused by ethanol treatmentBraz J Med Biol Res 1989 22(1):41-50. [Google Scholar]
[11]. Frommer DJ, The healing of gastric ulcers by zinc sulfateMed J Aust 1975 22(21):793-96. [Google Scholar]
[12]. Yazdanpanah K, Moghimi N, Yousefinejad V, Ghaderi E, Darvishi N, Effect of zinc sulphate on peptic ulcer diseasePak J Med Sci 2009 25(3):404-07. [Google Scholar]
[13]. Donma O, Donma MM, Sonmez S, Metal specification, phytochemicals and Helicobacter pylori infectionMed Hypoth 2006 67:545-49. [Google Scholar]
[14]. Handa O, Yoshida N, Tanaka Y, Ueda M, Ishikawa T, Takagi T, Inhibitory effect of polaprezinc on the inflammatory response to Helicobacter pyloriCan J Gastroenterol 2002 16(11):785-89. [Google Scholar]
[15]. Haristoy X, Fahey JW, Scholtus I, Lozniewski A, Evaluation of the antimicrobial effects of several isothiocyanates on Helicobacter pyloriPlanta Med 2005 71:326-30. [Google Scholar]
[16]. Vattem D, Lin Y, Shetty K, Enrichment of Phenolic Antioxidants and Anti- Helicobacter pylori properties of cranberry pomace by solid-state bioprocessingFood Biotechnology 2005 19(1):51-68. [Google Scholar]
[17]. Park S, Yeo M, Jin J, Lee K, Jung J, Choue R, Rescue of Helicobacter pylori–induced cytotoxicity by red ginsengDig Dis Sci 2005 50(7):1218-27. [Google Scholar]
[18]. Pories W, Henzel J, Rob C, Strain W, Acceleration of wound healing in man with zinc sulphate given by mouthThe Lancet 1967 289(7482):121-24. [Google Scholar]
[19]. Bandyopadhyay B, Banerjee P, Bhattacharya B, Bandyopadhyay SK, Serum zinc level: apossible index in the pathogenesis of peptic ulcer syndromeBiochem Mol Biol Int 1995 36(5):965-72. [Google Scholar]
[20]. Erzinkian KL, Lukasheva MV, Gobedzhavshili SD, Trapkov VA, Galaeva EV, Zinc metabolism in duodenal ulcerRoss Med Zh 1992 (5-12):14-15. [Google Scholar]
[21]. Mann NS, Mann SK, Brawn PN, Weaver B, Effect of zinc sulfate and acetylcysteine on experimental gastric ulcer: in vitro studyDigestion 1992 53(1-2):108-13. [Google Scholar]
[22]. Dsouza RS, Dhume VG, Gastric cytoprotectionIndian J Physiol Pharmacol 1991 35(2):88-98. [Google Scholar]
[23]. García-Plaza A, Arenas JI, Belda O, Diago A, Domínguez A, Fernández C, A multicenter clinical trial. Zinc acexamate versus famotidine in the treatment of acute duodenal ulcer. Study Group of Zinc acexamate (new UP doses)Rev Esp Enferm Dig 1996 88(11):757-62. [Google Scholar]
[24]. Troskot B, Simicevic V, Dodig M, Rotkvic I, Ivankovic D, Duvnjak M, Endogenous zinc concentrations in cysteamine-induced duodenal ulcers in the ratBiometals 1996 9(4) [Google Scholar]
[25]. Barazandeh F, Yazdanbod A, Pourfarzi F, Sepanlou SG, Derakhshan MH, Malekzadeh R, Epidemiology of peptic ulcer disease: endoscopic results of a systematic investigation in iranMiddle East J Dig Dis 2012 4(2):90-96. [Google Scholar]
[26]. Xia B, Xia HH, Ma CW, Wong KW, Fung FM, Hui CK, Trends in the prevalence of peptic ulcer disease and Helicobacter pylori infection in family physician-referred uninvestigated dyspeptic patients in Hong KongAliment Pharmacol Ther 2005 22(3):243-49. [Google Scholar]
[27]. Wong SN, Sollano JD, Chan MM, Carpio RE, Tady CS, Ismael AE, Changing trends in peptic ulcer prevalence in a tertiary care setting in the Philippines: a seven-year studyJ Gastroenterol Hepatol 2005 20:628-32. [Google Scholar]
[28]. Dong WG, Cheng CS, Liu SP, Yu JP, Epidemiology of peptic ulcer disease in Wuhan area of China from 1997 to 2002World J Gastroenterol 2004 10:3377-79. [Google Scholar]
[29]. Li Z, Zou D, Ma X, Chen J, Shi X, Gong Y, Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in ChinaAm J Gastroenterol 2010 105(12):2570-77. [Google Scholar]
[30]. Sung JJ, Kuipers EJ, El-Serag HB, Systematic review: the global incidence and prevalence of peptic ulcer diseaseAliment Pharmacol Ther 2009 29(9):938-46. [Google Scholar]
[31]. Aro P, Storskrubb T, Ronkainen J, Bolling-Sternevald E, Engstrand L, Vieth M, Peptic ulcer disease in a general adult population: the Kalixanda study: a random population-based studyAm J Epidemiol 2006 163(11):1025-34. [Google Scholar]
[32]. Zagari R, Law G, Fuccio L, Pozzato P, Forman D, Bazzoli F, Dyspeptic symptoms and endoscopic findings in the community: the loiano–monghidoro studyAm J Gastroenterol 2009 105(3):565-71. [Google Scholar]
[33]. Kargar M, Baghernejad M, Doosti A, Evaluation ofmetronidazole resistance of Helicobacter pylori strains. isolated from ShahrekordMed Sci J Tehran Azad Uni 2009 193:193-96. [Google Scholar]
[34]. Rasmi Y, Sadreddini M, Shahsavari Z, Raeisi S, Prevalence of Helicobacter pylori and cytotoxin-associated gene a in iranian patients with non-erosive and erosive reflux diseaseIndian Journal of Medical Sciences 2009 63(9):402 [Google Scholar]
[35]. López-Vidal Y, Ponce-de-León S, Castillo-Rojas G, Barreto-Zúñiga R, Torre-Delgadillo A, High diversity of vaca and caga helicobacter pylori genotypes in patients with and without gastric cancerPLoS ONE 2008 3(12):e3849 [Google Scholar]
[36]. Chiarini A, Calá C, Bonura C, Gullo A, Giuliana G, Peralta S, Prevalence of virulence-associated genotypes of Helicobacter pylori and correlation with severity of gastric pathology in patients from western Sicily, ItalyEur J Clin Microbiol Infect Dis 2008 28(5):437-46. [Google Scholar]
[37]. Dousti A, Ghorbani S, Karegar M, Epidemiology of bacterial infections in patients with gastrointestinal disordersJournal of Fasa University of Medical Sciences 2012 2(4):266-72. [Google Scholar]
[38]. Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB, Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimatedAm J Gastroenterol 1999 94(7):1834-40. [Google Scholar]
[39]. Meucci G, Battista R, Abbiati C, Benassi R, Bierti L, Bortoli A, Prevalence and Risk Factors of Helicobacter pylori-negative Peptic UlcerJournal of Clinical Gastroenterology 2000 31(1):42-47. [Google Scholar]
[40]. Jang H, Choi M, Shin W, Kim K, Chung Y, Kim K, Has peptic ulcer disease changed during the past ten years in korea? a prospective multi-center studyDig Dis Sci 2007 53(6):1527-31. [Google Scholar]
[41]. Groenen M, Kuipers E, Hansen B, Ouwendijk R, Incidence of duodenal ulcers and gastric ulcers in a western population: back to where it startedCanadian Journal of Gastroenterology 2009 23(9):604-08. [Google Scholar]