Pancreatic surgeries are usually performed for inflammatory conditions and neoplasms. In a study done on 172 pancreatic resection specimens, 65% of the cases were inflammatory lesions, 30% were neoplasms and the remaining 5% were non inflammatory non neoplastic lesions [1]. Amongst the neoplasms, western data suggest that ductal adenocarcinomas and its variants comprise more than 90% [2]. Intraductal Papillary Mucinous Neoplasm (IPMN) account for 5-7% of all pancreatic neoplasms [3–5]. IPMN is defined as a grossly visible mass forming, predominantly papillary non-invasive mucin producing epithelial neoplasm arising in the main pancreatic duct or branch ducts [3,6–8]. The minimum criterion to distinguish IPMNs from incidental microcysts and large pancreatic intraepithelial neoplasms (PanINs) requires the involved ducts to be more than 1cm in diameter [3]. It usually arises in the seventh to eighth decades of life [4,9]. They are typically associated with mucin accumulation within the lumen and cystic dilation of the ducts, which may be localized or involve the entire ductal system [4]. Microscopically, mucinous cells with various degrees of dysplasia (low, intermediate and high grade) line the ducts [10,11]. IPMNs are classified into four histopathological types based on the cytoarchitectural features and immunophenotype: gastric, intestinal, pancreatobiliary and oncocytic IPMNs, accounting for 49-63%, 18-36%, 7-18% and 1-8% respectively [5,9].
The aim of our study was to assess the spectrum of different pancreatic pathologies on pancreatic resection specimens. The second objective was to review and share our experience on the clinico-pathological features of IPMNs in our institute. This study was taken up as there has been no published data available in Indian context to the best of our knowledge.
Materials and Methods
This was a retrospective study of all cases where pancreatic surgeries were done for pancreatic pathologies March 2002 to February 2016 in a tertiary care center. The pathology report details of these pancreatic pathologies were obtained from the departmental online database. The slides and blocks of diagnosed cases of IPMNs were retrieved from the department archives, reviewed and a detailed study on the histopathological features was done with recording of the data. The relevant clinical details were extracted from the online database which included the following: age, sex and presenting complaints. The laboratory parameters taken for evaluation in this study included serum levels of CA19-9 before surgery. Radiological findings of the Ultrasonogram (USG) and Computed Tomography (CT) (wherever available) were also noted from the online database with special emphasis on the location of the tumour including duct type, focality, and presence or absence of local or distant metastasis. Type of surgery done was noted. Macroscopically, the tumour location including duct type, number, diameter and length of the involved duct, appearance and size of mural nodule, and the presence or absence of necrosis within the tumour were recorded. Microscopic examination of the slides was done in detail and the histological parameters analysed included the pattern of arrangement of the tumour cells, histological subtype, grade of dysplasia and foci of invasion. At the time of data collection, the outcome status of these cases in the latest follow up with relevant imaging features were also recorded. The data was analysed using excel spread sheet and statistical analysis was done using simple descriptive statistics.
Results
In the 14 year study period, 377 pancreatic surgeries were performed which included cored out head specimens (92, 24.4%), Whipple’s pancreaticoduodenectomy specimens (73, 19.4%), distal pancreatectomies with splenectomy (73, 19.4%), necrosectomies (58, 15.4%), distal pancreatectomies without splenectomy (39, 10.3%), excision specimens (23, 6%), subtotal pancreatectomies (9, 2.3%), central pancreatectomies (8, 2%) and total pancreatectomy with splenectomy (1, 0.3%). The pathological diagnoses have been listed in [Table/Fig-1]. Pancreatitis was the most common diagnosis followed by exocrine neoplasms and endocrine tumours.
Histopathological diagnosis of 377 pancreatic surgeries.
| Diagnosis | N (%) |
|---|
| Pancreatitis | 165 (43.8) |
| Exocrine neoplasms | 126 (33.4) |
| Endocrine neoplasms | 64 (17) |
| Haemorrhage/Infarction (post trauma) | 12 (3.2) |
| Nesidioblastosis | 7 (1.82) |
| Hydatid cyst | 2 (0.52) |
| Tuberculosis | 1 (0.26) |
Among the 190 neoplasms [Table/Fig-2], endocrine neoplasms were the most common followed by solid pseudopapillary epithelial neoplasm (SPEN) and adenocarcinomas. There were 6 diagnosed cases of IPMNs [Table/Fig-3].
Histological spectrum of pancreatic neoplasms (190 cases).
| Diagnosis | N (%) |
|---|
| Endocrine tumours | 64 (33.7) |
| Solid pseudopapillary epithelial neoplasm (SPEN) | 46 (24.2) |
| Adenocarcinoma | 40 (21) |
| Mucinous cystic neoplasm | 18 (9.5) |
| Serous cystic neoplasm | 6 (3.2) |
| Intraductal papillary mucinous neoplasm (IPMN) | 6 (3.2) |
| Acinar cell carcinoma | 1 (0.52) |
| Mixed ductal endocrine carcinoma | 1 (0.52) |
| Lymphoma | 3 (1.6) |
| Metastasis | 2 (1) |
| Cystic lymphangioma | 1 (0.52) |
| Clear cell sarcoma of soft parts | 1 (0.52) |
| Ewing/Primitive neuroectodermal tumour (PNET) | 1 (0.52) |
Clinico-pathological profile of Intraductal papillary mucinous neoplasms (IPMNs).
| Case | Age | Sex | Location | Duct type | Histologic type | Dysplasia | Invasive carcinoma | Follow-up months | Follow-up |
|---|
| Case 1 | 53 | F | Head | Main | Gastric Foveolar | Intermediate | No | 1 | NED** |
| Case 2 | 48 | M | Tail & Body | Mixed | Pancreatobiliary | High | Yes | 30 | Liver metastasis and abdominal wall nodule at 18 months |
| Case 3 | 51 | M | Tail | Branch | Gastric Foveolar | Low | No | 22 | NED** |
| Case 4 | 41 | M | Head | Main | Gastric Foveolar | Low | No | 12 | NED** |
| Case 5 | 61 | M | Head | Main | Intestinal | Intermediate | No | 3 | NED** |
| Case 6 | 71 | M | Head | Branch | Intestinal | Intermediate | No | 1 | NED** |
*DPS – Distal pancreatectomy and splenectomy; **NED – No evidence of disease.
Epidemiology
IPMNs constituted 1.6% of all the pancreatic lesions, 3.2% of all pancreatic neoplasms and 4.8% of all pancreatic exocrine neoplasms. Male to female ratio was 5:1. Median age was 52 years (41-71 years).
Clinical Features and laboratory investigations
All patients presented with pain abdomen. One case also had features of obstructive jaundice. Two of the patients had a long standing history of smoking and one of them also had history of chronic alcohol intake. The median serum CA19-9 level was 61.75 U/ml (8.72 to 38000 U/ml).
Radiological Features and Surgeries Performed
The radiological features and surgeries performed are shown in [Table/Fig-4]. Two of the six patients also had a smaller concomitant lesion in the uncinate process. There was no evidence of local or distant metastasis.
Radiological details and surgeries performed on patients with Intraductal papillary mucinous neoplasms (IPMNs).
| Case | Location (main lesion) | Concomitant smaller lesion | Duct type | Surgery |
|---|
| Case 1 | Head | None | Main | Whipple |
| Case 2 | Tail & Body | Uncinate process | Mixed | DPS* with subtotal gastrectomy, omentectomy & limited colectomy |
| Case 3 | Tail | Uncinate process | Branch | DPS* |
| Case 4 | Head | None | Main | Whipple |
| Case 5 | Head | None | Main | Whipple |
| Case 6 | Head | None | Branch | Whipple |
*DPS – Distal pancreatectomy and splenectomy.
Whipple procedure or distal pancreatectomy with splenectomy (DPS) was performed in all the cases. In addition to DPS, a subtotal gastrectomy, omentectomy and limited colectomy was also performed in the 2nd patient (case 2) in view of peripancreatic adhesions.
Gross Pathology [Table/Fig-5]
a. Whipple specimen with a multiloculated mucin filled cystic tumour containing mural nodule located in the head of pancreas. b. Distal pancreatectomy and splenectomy specimen with a cystic tumour in the tail of pancreas (red arrows). The main pancreatic duct is mildly dilated (blue arrows).

All the tumours showed dilated ducts filled with mucin and with an attached mural nodule. Three were main duct type, 2 were branch duct type and the remaining one was of mixed duct type. The length of the main duct involved ranged from 5.5cm to 8.5cm. The diameter of the involved duct ranged from 1.1cm to 6cm. The diameter of the smallest mural nodule was 0.5cm and the largest was 3 cm. Three of the mural nodules had a solid grey white cut surface with visible papillary excrescences, 2 had a solid and cystic cut surface, and the remaining one had a friable cut surface (case 2). Case 2 also showed a separate solid tumour with a grey white cut surface in the adjacent pancreatic parenchyma with a maximum dimension of 0.8 cm. This lesion was identified after diligent grossing of the specimen.
Microscopic Pathology
Histologically, 3 cases (50%) were of gastric foveolar type, 2 (33%) were intestinal type and the remaining one (17%) was pancreatobiliary type. 2 cases (33%) showed low grade dysplasia (both gastric foveolar type), 3 cases (50%) showed intermediate grade dysplasia (2 intestinal and 1 gastric foveolar type) and 1 case (17%) showed high grade dysplasia (pancreatobiliary type).
In the gastric foveolar type (cases 1, 3 & 4) [Table/Fig-6], the ducts were lined by columnar epithelial cells with basally located uniform nuclei and abundant amounts of vacuolated cytoplasm containing mucin (low grade dysplasia). In one of the cases, the lining epithelium showed foci of mildly pleomorphic nuclei with prominent nucleoli and displaying loss of polarity (intermediate grade dysplasia). This case also had focal intestinal type of epithelium with admixed goblet cells. The adjacent pancreatic parenchyma showed changes of chronic pancreatitis and no evidence of pancreatic intraepithelial neoplasia or invasive malignancy.
IPMN, gastric foveolar type with low grade dysplasia. a. Dilated duct lined by mucinous columnar epithelium with a basally located uniform nuclei resembling gastric foveolar cells. H&E x100. b. The cells containing abundant mucin. d-PAS x100.

In the intestinal type (case 5 & 6) [Table/Fig-7], the ducts were lined by mucinous columnar epithelial cells which were thrown into villous projections and containing mildly pleomorphic nuclei displaying pseudostratification, loss of polarity and occasional mitosis (intermediate grade dysplasia). Admixed goblet cells were seen. Both the cases also had gastric foveolar type of epithelial lining in foci. The adjacent pancreatic parenchyma showed changes of chronic pancreatitis and no evidence of pancreatic intraepithelial neoplasia or invasive malignancy.
Intraductal papillary mucinous neoplasm (IPMN), intestinal type with intermediate grade dysplasia. a. Duct lined by villiform structures lined by columnar epithelial cells with occasional admixed goblet cells. H&E x100. b. Columnar epithelial cells with moderately pleomorphic nuclei, prominent nucleoli and displaying occasional mitotic activity. H&E 200x. c. Columnar epithelial cells with admixed goblet cells. d-PAS x200.

In the pancreatobiliary type (case 2) [Table/Fig-8], the ducts were lined by complex and arborising papillary structures lined by columnar epithelial cells with apical mucin. The cells had a moderately pleomorphic nuclei displaying pseudostratification and increased mitotic activity (high grade dysplasia). The adjacent pancreatic parenchyma showed chronic pancreatitis and a focus of invasive ductal type adenocarcinoma. The resected part of stomach and colon showed areas of serosal fibrosis and chronic inflammation and no definite tumour infiltration.
IPMN, pancreatobiliary type with high grade dysplasia. a. Duct with complex papillary and tubular structures lined by columnar epithelial cells. H&E 100x. b. Columnar epithelial cells lined by moderately pleomorphic nuclei displaying loss of polarity, stratification and frequent mitosis (red arrows). H&E 400x
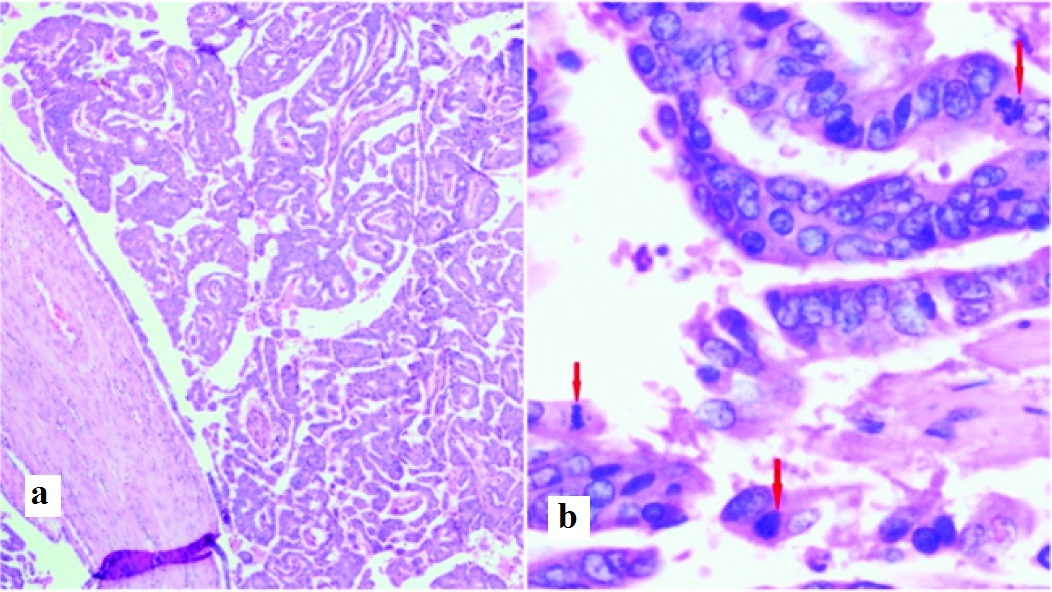
Follow-up [Table/Fig-3]
All the 5 cases of intestinal type and gastric foveolar type had no evidence of recurrent tumour or metastasis. The pancreatobiliary type (case 2) had evidence of liver metastasis (radiology) and abdominal wall metastases, 18 months after initial diagnosis. The concomitant cystic lesion which was seen in the head of pancreas in case 2, which was not operated initially, had enlarged to 10.5cm in maximum dimension (radiology) in the last follow up. In case 3, the lesion in head of pancreas remained the same without any evidence of progression.
Discussion
In our study, pancreatic neoplasms were more common than inflammatory conditions. This was in contrast to the findings seen in the study done by Habanec et al., [1]. This could be attributed to increased radiological detection of surgically resectable neoplasms. Amongst the neoplasms, endocrine neoplasms, SPEN, ductal adenocarcinoma and IPMN constituted approximately 34%, 24 %, 21% and 3.2 % respectively. The western data suggest that ductal adenocarcinomas and its variants comprise more than 90% of all pancreatic neoplasms [2]. The low percentage of pancreatic ductal adenocarcinoma in our study could be due to the fact that many such patients probably presented to the hospital at an advanced and inoperable stage.
In the current study, IPMN constituted 3.2% of all pancreatic neoplasms whereas in the West, slightly higher rates 5-7% have been observed [3–5]. Amongst the exocrine neoplasms alone, IPMN represented 4.8% in this study, while in the West, it comprised 3% [3].
IPMNs occurred in men more frequently than women with a male: female ratio of 5:1 which has been described in the literature [9]. Patients had a median age of 6th decade with similar observations seen in the studies done by Angelica et al., Kang et al., and Schaberg et al., [12–14]. They usually present with vague abdominal pain [4,9,11,12,14].
Approximately 70-80% of IPMNs occur in the head/uncinate process of the pancreas with similar observations seen in the current study (60-70%) [4,9].
IPMN can manifest as multilocular cystic masses or abundant papillary nodules. Papilla formation may be seen microscopically or macroscopically [4]. Papillary excrescences were seen in 2 of our cases macroscopically and all 6 cases microscopically. They are currently classified based on their macroscopic appearance, grade of dysplasia and histological cell type [4,9,11].
Based on the extent of involvement of the ductal system, IPMNs are classified as main duct (16-36%) and branch duct types (40-65%). Mixed duct type (15-23%) is a combination of the main duct and branch duct type [4,11]. In the current study, main duct type was seen in 50% of cases, branch duct in 33% and mixed duct in 17%. Branch duct IPMNs may appear as multiple, separate, smaller cysts macroscopically and radiologically because of the tortuous dilated ducts [4,10].
IPMNs are classified into four histopathological types: gastric, intestinal, pancreatobiliary and oncocytic IPMNs based on the cytoarchitectural and immunophenotypical features [5,9]. The gastric-type IPMNs show relatively simple and typically short papillae and often have pyloric glands in the cyst wall. The epithelial lining is identical to gastric foveolar epithelium. The intestinal-type IPMN typically has a villous growth pattern and cytology similar to that of villous adenoma in the colon. It shows pseudostratified columnar cell lining with a basophilic appearance and apical goblet-like mucin. The pancreatobiliary type typically has complex arborizing and interconnecting papillary configurations with thin fibrovascular cores and is composed of cuboidal cells with enlarged nuclei and scanty mucin production. The oncocytic type also has a complex arborizing papillary architecture and intraepithelial cribriform formations but with a distinctive eosinophilic cytoplasm. The current WHO 2010 classification puts this intraductal oncocytic papillary neoplasm (IOPN) under the general category of IPMN [3,11].
In the current study, 3 (50%) were gastric foveolar types, 2 (33%) were intestinal types, and the remaining 1 (17%) was pancreatobiliary type. We had no oncocytic type. Similar observations were noted in the studies done by Ferrone et al., and Castellano et al., wherein the gastric foveolar type, intestinal type, pancreatobiliary type and oncocytic type accounted for 49-63%, 18-36%, 7-18% and 1-8% respectively [5,10].
Low-grade dysplasia is characterized by a uniform monolayer of columnar cells with a basally located nuclei showing no or minimal atypia. In the intermediate-grade of dysplasia, there is higher nuclear atypia in the form of nuclear pleomorphism, nuclear enlargement and pseudostratification. In high-grade dysplasia, there is marked cytological atypia and complex architecture with many cribriform groups and micropapillary tufting and budding of neoplastic cells into the lumen [10,11].
This spectrum, previously was classified as “hyperplasia -adenoma – borderline – in situ carcinoma – invasive carcinoma” and later as “low-grade dysplasia – intermediate-grade dysplasia – high-grade dysplasia – invasive carcinoma” and is now being reclassified as “low-grade dysplasia – high-grade dysplasia – invasive carcinoma” as per the Baltimore consensus [3,15].
In the current study with respect to dysplastic changes, 2 cases (33%) were low grade, 3 cases (50%) were intermediate grade and 1 case (17%) was high grade. The study by Kang et al showed 18% to have low grade, 45.5% to have intermediate grade and 8.5% to have high grade dysplasia [13].
The overall incidence of an associated invasive carcinoma in IPMNs is relatively low and had been noted in 28% of cases in Kang et al., series [13]. In our current study, only one case (17%) had invasive adenocarcinoma. The prevalence of invasive adenocarcinoma in branch duct IPMNs of the intestinal and pancreatobiliary types is lower (15%) as compared to the main duct IPMNs of the same types (30%) [11]. Invasive carcinoma is rarely found in gastric foveolar type [11].
Meticulous sampling of the specimen is necessary for detection of an invasive carcinoma. Invasive carcinoma, is usually a colloid or tubular (conventional ductal) type. Colloid carcinoma is usually found in intestinal type, and tubular carcinoma is found in the pancreaticobiliary type [11].
The presence of an invasive component in the tumour is a significant predictor of poor survival outcome [11,12]. Recent studies have uniformly shown that half of those IPMNs with an associated invasive carcinoma died from their disease whereas the vast majority of the completely resected non-invasive IPMNs have a 5-year survival outcome of more than 90% [4,7,12].
The background pancreatic parenchyma showed features of chronic pancreatitis in all cases, as described in literature [4]. The limitations of this study are that it included a very small sample size (6 cases) over a 14-year study period in view of rarity of these neoplasms.
Conclusion
IPMNs are rare neoplasms of pancreas with a male predominance. They are usually indolent except for the pancreatobiliary type which may have an aggressive course and often associated with an invasive adenocarcinoma component. Thorough sampling of the specimen is necessary for detection of an invasive carcinoma. Diligent follow-up is recommended.
*DPS – Distal pancreatectomy and splenectomy; **NED – No evidence of disease.