Periodontitis is primarily a bacterial infection caused by diverse group of microorganisms, which has also been stated as the sixth complication of diabetes mellitus by Loe et al., [1,2]. Though microbes are implicated as the etiological agents that bring about the inflammatory lesion, chemical mediators of inflammation which are produced both by the microbes as well as the host inflammatory responses play a vital role in the loss of connective tissue as well as supporting alveolar bone [3]. Some of the established chemical mediators include the various pro-inflammatory cytokines like IL-1 and TNF-α [4]. These chemical mediators aid to enhance the ongoing inflammatory process that is initiated by the microbes thereby resulting in disease progression and hence loss of supporting periodontal tissues. Also because the levels of these chemical mediators vary with the disease progression many of these have been suggested to be used as biomarkers for assessing periodontal disease activity [5]. One such chemical mediator which has been linked with periodontal disease in the recent past and has already been associated with T2DM is visfatin [6].
Visfatin is named so because it is derived from the word “VISceral FAT”. Though it is preferentially produced in the visceral adipose tissue it can be expressed in the isolated subcutaneous adipose tissue as well [7]. It can also be found in the skeletal muscle, liver, bone marrow and lymphocytes [8]. Elevated plasma levels of visfatin have been reported in a group of patients with T2DM on hypoglycemic treatment [9]. Also in a recent study by Pradeep et al., elevated levels of visfatin have been identified in the individuals with periodontal disease when compared to healthy subjects [6]. It has been attributed in roles such as enhancement of neutrophil apoptosis and by itself it can be enhanced by the secretion of various pro-inflammatory cytokines like TNF-α and IL-6 [10,11].
Diabetes mellitus is a disease of defective metabolism associated with elevated blood glucose levels which itself is associated with various micro and macrovascular complications [2]. Though the various pathways by which diabetes and periodontitis are linked have been reported in the past there are several mechanisms that still needs to be explored. Of the biochemical parameters adipocytokines are released from the adipose tissues and have been linked with diabetes in causing insulin resistance and have also been linked with increased periodontal disease progression. As per the available literature various adipocytokines that have been linked with the disease include TNF-α, IL-6, leptin, adiponectin, resistin and recently visfatin [12–14]. Due to its pathophysiologic functions visfatin can add on to the understanding of increased periodontal destruction in this disease if present in an elevated level in the oral fluids of diabetics.
Thus, the present study was conducted with an attempt to identify any such adding interlink between T2DM and periodontitis and we aimed to identify the: a) levels of visfatin in systemically and periodontally healthy subjects, b) in systemically healthy subjects with chronic periodontitis before and after non surgical periodontal therapy, c) in controlled type 2 diabetics with chronic periodontitis before and after non-surgical periodontal therapy and d) to identify the correlation between these groups if any.
Materials and Methods
Forty two subjects with age range of 30-65 years visiting the Department of Periodontics and Oral Implantology SDM College of Dental Sciences and Hospital, Dharwad, Karanataka, india were recruited in this longitudinal study and were divided into three groups with 14 subjects in each group. Plaque scores were taken for sample size calculation for the study. The power of the test was set at 80% with a two sided 5% significance level. The formula used for sample size calculation was as follows:
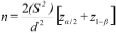
Following which a total of 42 subjects were recruited. The duration of study was 30 days after which the subjects were recalled.
For inclusion the subjects were considered if they showed the characteristic criteria of disease as per each group as described below, and only when they had at least 20 natural teeth present.
Subjects with aggressive periodontitis, use of tobacco in any form, gross oral pathology, tumours, or any other systemic disease that can alter the course of periodontal disease except controlled T2DM, those who had taken medication affecting periodontal status or had received periodontal therapy in the preceding six months were excluded from the study.
The groups were as follows
GROUP 1 (Healthy): Subjects with clinically healthy periodontium, and also with no signs and symptoms of systemic disease.
GROUP 2 (Chronic periodontitis): Subjects with chronic periodontitis as per AAP 1999 criteria, but with no signs and symptoms of systemic disease.
GROUP 3 (Chronic periodontitis with type 2 diabetes mellitus): Subjects with chronic periodontitis as per AAP 1999 criteria and with controlled T2DM as determined by Glycated Hemoglobin (HbA1c < 7), but with no other diabetic complications.
Parameters recorded for all the groups were, Gingival Index (GI), Plaque Index (PI) Probing Pocket Depth (PPD) Glycated Hemoglobin (HbA1c) levels and Body Mass Index (BMI) at both baseline and at recall interval of 30 days for all the three groups. Scaling and root planing was performed for Group 2 and 3, whereas since Group 1 constituted of systemically and periodontally healthy subjects no treatment was done for this group.
A mouth mirror and explorer was used for the estimation of GI and PI. PCP-UNC 15 probe with markings till 15mm and calibrations accurate uptill 1mm was used for the measurement of periodontal pockets. NycoCard® HbA1C test kit (AXIS-SHIELD, Oslo, Norway) was used to evaluate glycated hemoglobin levels.
Anthropometric measurements included weight (kg) and height (m) of the patients to calculate the BMI (weight divided by the square of height, kg/m2). Obesity was defined as BMI ≥ 30 while being overweight was defined as BMI 25 – 29.9 and normal weight was defined as BMI ranging from 20 to 24.9 kg/m2 [15].
Microcapillary tubes with a standard diameter and length marked up till 5microlitres obtained from Top-Tech biomedicals, Mumbai were used for collecting a standard volume of 3μl gingival crevicular fluid (GCF). The obtained fluid was transferred using 3-way air pressure syringe into safe lock eppendorf tubes containing a standard of 100μl of Phosphate Buffer Saline (PBS). The collection of GCF followed the extra-crevicular non-stimulatory method. The sites selected for collection of GCF were dried using a three way air syringe and isolated with cotton roles to prevent contamination with saliva. In order to prevent contamination, the supragingival deposits were removed using a sterile curette. Calibrated, volumetric microcapillary pipette were placed at the entrance of the sulcus. A standard volume of 3μl of GCF was collected. Micropipettes that were contaminated with blood were discarded.
An ELISA kit for the identification of visfatin in the collected samples was obtained from the Genexbio Company, New Delhi, India. The kit was stored at a temperature of -800C till all the samples were collected. Visfatin was estimated from the GCF using Sandwich ELISA technique. The kit had polyclonal antibodies against visfatin with a minimum detection level of 379pg/ml (0.379ng/ml). At the end of the ELISA procedure a colored product was obtained the intensity of which was measured at 450nm absorbimetery range using LISA microplate reader. The intensity of color was inversely proportional to the concentration of visfatin in the samples.
Ethics
An ethical clearance was obtained from the institutional review board and an informed written consent was obtained from all the subjects before their participation in the study. It was in accordance with the Helsinki declaration of 1975 which was revised in year 2000.
Statistical Analysis
The statistical analysis was carried out using SPSS 20 software. Inter-group comparisons were carried out using one way ANOVA and Tukey’s post hoc procedures analysis. Intra-group comparisons were made using Wilcoxon matched pairs test. Karl Pearson’s correlation coefficient was used to evaluate the correlation of visfatin with other clinical parameters.
Results
A total of 42 individuals 18 males and 24 females in the age range of 30-65 years participated in the study; they were divided into three groups as explained in the material and method section. A standard of 3μl GCF was collected from all the individuals to ensure that the levels of visfatin do not vary due to difference in volume of GCF. Of the participants 18 were males and 24 were females. All the subjects fell within the age range of 30-65 years [Table/Fig-1]. The results of the six parameters at baseline and at the end of one month are presented.
Demographic data of the groups as per age and gender.
| Group | Male | % | Female | % | Total subjects | Mean age | Standard deviation |
|---|
| G1 | 3 | 21.43 | 11 | 78.57 | 14 | 32.43 | 2.03 |
| G2 | 8 | 57.14 | 6 | 42.86 | 14 | 41.71 | 8.06 |
| G3 | 7 | 50.00 | 7 | 50.00 | 14 | 48.07 | 5.54 |
| Total | 18 | 42.86 | 24 | 57.14 | 42 | 40.74 | 8.60 |
Mean HbA1c scores of Group 3 was significantly higher than that of Group 1 and Group 2. Also no statistical difference was seen on comparison of Group 1 and 2 scores [Table/Fig-2]. The mean PI scores in Group 1 were significantly lower when compared to both the periodontally diseased groups. At the end of one month period the scores decreased significantly compared to the pre-treatment values in both Group 2 and Group 3, but on inter-group comparison it remained statistically insignificant. No changes were seen in Group 1 scores at the follow-up visit. The mean scores for GI, PPD and visfatin in Group 1 were significantly lower when compared to both the periodontally diseased groups. Also a significant difference between Group 2 and Group 3 was seen with Group 3 having the highest mean score for all the parameters.
Inter-group comparison of HbA1c (%) using One way ANOVA and Tukey’s multiple post hoc procedures.
| Group | Mean | SD |
|---|
| G1 | 4.78 | 0.52 |
| G2 | 4.71 | 0.59 |
| G3 | 6.71 | 0.17 |
| Total | 5.40 | 1.04 |
| F-value | 84.5963 | |
| p-value | 0.00001* | |
| Pair wise comparison by Tukey’s post hoc procedures |
| G1 vs G2 | p=0.9284 |
| G1 vs G3 | p=0.0001* |
| G2 vs G3 | p=0.0001* |
*p<0.05
At the end of one month period the visfatin scores decreased significantly compared to the pre-treatment values in both Group 2 and Group 3, while no changes were seen in Group 1 subjects [Table/Fig-3], but on inter-group comparison of post-treatment levels it still remained significantly more in Group 3 when compared to Group 2 despite of the same treatment and follow-up duration in both the groups. No changes were seen in Group 1 scores at the follow up visit as mentioned [Table/Fig-4].
Intra group comparison of baseline and one month visfatin (ng/ml) levels using Wilcoxon matched pairs test.
| Group | Time | Mean | SD | Mean Diff. | SD Diff. | % of change | Paired t | p-value |
|---|
| G1 | Baseline | 9.29 | 3.68 | 0.03 | 0.08 | 0.31 | 1.3512 | 0.1997 |
| 1-month | 9.26 | 3.66 |
| G2 | Baseline | 71.48 | 8.90 | 50.33 | 5.61 | 70.40 | 33.5865 | 0.00001* |
| 1-month | 21.16 | 4.88 |
| G3 | Baseline | 88.81 | 6.39 | 59.38 | 7.15 | 66.86 | 31.0603 | 0.00001* |
| 1-month | 29.43 | 4.32 |
*p<0.05
Inter-group comparison for baseline and one month post-treatment Visfatin (ng/ml) scores using Kruskal Wallis ANOVA and Tukey’s multiple post hoc procedure.
| Group | Baseline | One month | Difference |
|---|
| Mean | SD | SE | Mean | SD | SE | Mean | SD | SE |
|---|
| G1 | 9.29 | 3.68 | 0.98 | 9.26 | 3.66 | 0.98 | 0.03 | 0.08 | 0.02 |
| G2 | 71.48 | 8.90 | 2.38 | 21.16 | 4.88 | 1.31 | 50.33 | 5.61 | 1.50 |
| G3 | 88.81 | 6.39 | 1.71 | 29.43 | 4.32 | 1.15 | 59.38 | 7.15 | 1.91 |
| F-value | 549.6164 | 77.2446 | 519.8216 |
| p-value | 0.00001* | 0.00001* | 0.00001* |
| Pair wise comparison by Tukey’s post hoc procedures |
| G1 vs G2 | p=0.0001* | p=0.0001* | p=0.0001* |
| G1 vs G3 | p=0.0001* | p=0.0001* | p=0.0001* |
| G2 vs G3 | p=0.0001* | p=0.0001* | p=0.0003* |
*p<0.05
The BMI status did not vary in all three groups significantly. Using Karl Pearson’s correlation coefficient, a statistically significant correlation of visfatin concentration was obtained with GI, PI, PPD, HbA1c and BMI levels when the samples were grouped together and correlated overall [Table/Fig-5].
Correlation of Visfatin levels with GI, PI, PPD and HbA1c, BMI by Karl Pearson’s correlation coefficient (overall samples at baseline).
| Variables | Visfatin levels |
|---|
| Correlation coefficient | p-value |
|---|
| GI | 0.923 | <0.001* |
| PI | 0.541 | <0.001* |
| PPD | 0.932 | <0.001* |
| HbA1c | 0.587 | <0.001* |
| BMI | 0.658 | 0.7297 |
*correlation is significant at 0.05 level
Discussion
Visfatin was previously characterized as the Pre-B-Cell Colony Enhancing Factor (PBEF) involved in the differentiation of B cells, however it has been shown to play many other roles as well [16]. Fukuhara et al., in his in vitro study linked it with T2DM reporting it to function similar to insulin, later the mechanism behind such an action was explored and also clinical studies reporting an increase of visfatin levels in T2DM were published [17]. Besides this simultaneous studies conducted on visfatin also revealed its role in the inflammatory process [9,10].
It has been associated with many inflammatory diseases including Rheumatoid arthritis, sepsis, acute lung injury and atherosclerosis [18–20] etc. This correlation can be explained by the fact that it has been known to function in co-ordination with the pro-inflammatory cytokines, IL-6, IL-1β, and TNF-α have been shown to increase its expression significantly [11]. Also it plays a role in the persistence of inflammation by its capacity to inhibit the neutrophil apoptosis [10]. Also, elevated levels of visfatin have been shown in the GCF of periodontally diseases subjects. Since, visfatin has been shown to be increased in subjects with T2DM the levels of visfatin have been evaluated in a cross-sectional study in the GCF of periodontally diseased individuals with T2DM where a positive association was found [21,22]. Though whether an increase in visfatin in the GCF of periodontally diseased individuals with T2DM would change after periodontal therapy has not been evaluated as per our knowledge.
Thus, the present study (which is one of the few studies involving visfatin, periodontitis, and T2DM) was conducted to estimate and compare the concentration of visfatin from the GCF of healthy and periodontitis subjects with or without T2DM, to analyze the impact of an inflammatory state and an altered glycemic state individually and simultaneously. An increase in systemic inflammation, oxidative stress and periodontal destruction has been established as an outcome of T2DM [23–25]. Also T2DM is a known risk factor for periodontal disease [21] thus the levels of visfatin were decided to be checked in these subjects. HbA1c levels of known type 2 diabetics were recorded in order to assess the degree of glycemic controls of the subjects and only those with HbA1c < 7 categorized as controlled diabetics [26] were planned to be included in the study, all the subjects of Group 3 in this study had HbA1c in the range of 6.5-6.9.
Well-controlled diabetics were selected since such subjects have shown to possess no significant increase in the risk for periodontitis and for longitudinal bone loss compared to non-diabetic controls [27]. It was interesting to observe that though the diabetics were controlled the range of HbA1c for Group 3 was 6.5-6.9 which was significantly higher than both the non-diabetic groups Group1 and Group 2 where it was 3.9-5.7 and 3.9-5.4 respectively. The mean HbA1c levels of the diabetic subjects in the present study were similar to the HbA1c levels of diabetics in a study conducted by Lopez et al., where it was 6.9 ± 0.12. In the same study the authors found the circulating visfatin levels to be highly correlated with the HbA1c levels, which they stated could be due to a greater β-cell destruction which occurs with elevated levels. Thus, despite the subjects in Group 3 being controlled diabetics a significantly increased HbA1c in them when compared to the systemically healthy individuals may have contributed to greater visfatin levels in these individuals [28]. Also diabetics are subjected to a state of sub-clinical inflammation and altered lipid metabolism due to compromised insulin activity which can lead to a resultant increase in visfatin irrespective of their glycemic control [29]. Controlled type 2 diabetes mellitus independently has shown to have elevated serum Visfatin levels [28] and also an increase in the GCF visfatin levels in such individuals has been shown when compared with systemically healthy controls by Pradeep et al., [22]. Thus, an overall increase in Group 3 which was more than Group 1 or Group 2 can also be attributed to the systemic disease. Elevated levels of visfatin in diabetics could be because of, subclinical inflammation, higher triglyceride levels which been stated to be independently associated with increased visfatin levels, impaired levels of glucocorticoids which in turn enhances its release from adipocytes [30], and a greater β-cell impairment due to significantly higher HbA1c levels.
Duration of study for the present study was 30 days, which is the minimum time period required for the inflammatory parameters to reduce after non-surgical periodontal therapy. Subjects of Group 3 presented with highest levels of PI, and GI post-treatment and also showed the highest concentration of visfatin. Statistically significant decrease in levels of visfatin was seen one month post treatment in Group 2 and Group 3 after scaling and root planning (SRP) in comparison with baseline. The decrease in visfatin levels in Group 2 was in accordance with the results of Raghavendra et al., [31].
Relation of visfatin with BMI and abdominal fat is questionable. Since there is evidence to show that visfatin increases with obesity [32], BMI was recorded for all subjects in order to avoid any variations due to it, which did not show any significant variations amongst the three groups.
In the present study, an overall correlation analysis of visfatin levels with different clinical parameters showed a significant correlation with respect to GI, PI, PPD, HbA1c and BMI levels.
Although the overall visfatin levels in GCF were found to be strongly correlating with GI, PI, PPD, HbA1c and BMI it was interesting to observe that there was weak correlation of visfatin levels to each of the study groups with respect to GI, PI, PPD, HbA1c and BMI. The affected weak correlation may be attributed to the extreme values of estimated visfatin levels in GCF. However, there was a strong correlation of visfatin levels with PPD in Group 3. Though this warrants a careful interpretation of all results with respect to the association of visfatin levels with the concerned variables, the positive association of visfatin levels with PPD in Group 3 makes it obvious that it has a role in pathogenesis of periodontitis and type 2 diabetes mellitus as two interconnected diseases, since the PPD is an inflammatory variable. The amount and severity periodontal tissue destruction is influenced by T2DM and such an association has been documented [21,33].
In this study a positive correlation that was observed in the overall samples between BMI and visfatin levels is in contrast to the results of Esteghamati et al., where visfatin in type 2 diabetics was found to be elevated independently of BMI and visceral fat [30].
This study used the PPD as a parameter for assessment since a manual probing method was used. However, the measurement of the clinical attachment loss is a more accurate method for assessing the changes in the inflammatory disease and thus it is one of the limitations of this study.
Since Type 2 diabetes mellitus and periodontal disease has already been established as a two way association, this adipocytokine adds on to our understanding of the pathogenesis of this two-way relation, and also clearly helps the clinician understand the importance of keeping the inflammation of the periodontium under control for a better diabetic control.
It is therefore, with caution stated that visfatin levels in GCF show an overall positive correlation to all the variables assessed in this study, in spite of a general contradictory association when analyzed group-wise, with one exception. Also, a categorical statement that visfatin has a place in the etiopathogenesis of periodontitis and T2DM is evidenced in the literature and this study provides an implication that visfatin is associated with inflammatory and tissue destruction variables of periodontal disease as well as variables of T2DM.
Conclusion
Thus, within the limitations of this study it can be concluded that visfatin is present in the GCF of all subjects irrespective of their health status, though the levels are higher in the peridontally diseased subjects. The levels of visfatin are also increased in subjects with both periodontal disease and T2DM, the levels being highest in this group which decrease with periodontal treatment.
Since in our study we found an overall correlation of visfatin with other clinical parameters including PI, GI, PPD and HbA1c we may conclude that visfatin may have a role to play in pathogenesis of periodontitis. Also, since visfatin has been related with an increase in inflammatory factors elevated levels of visfatin in Type 2 diabetics may explain one of the mechanisms of increased risk of periodontal disease in these subjects.
*p<0.05
*p<0.05
*p<0.05
*correlation is significant at 0.05 level