Immunohistochemical Detection of p75 Neurotrophin Receptor (p75-NTR) in Follicular and Plexiform Ameloblastoma
Yoithapprabhunath Thukanayakanpalayam Ragunathan1, Nirmal Ramadas Madhavan2, Sunil Paramel Mohan3, Srichinthu Kenniyan Kumar4
1 Reader, Department of Oral and Maxillofacial Pathology, Vivekanandha Dental College for Women, Tamilnadu, India.
2 Professor and Head, Department of Oral and Maxillofacial Pathology, Rajah Muthiah Dental College and Hospital, Tamilnadu, India.
3 Professor and Head, Department of Oral and Maxillofacial Pathology, Sree Anjaneya Institute of Dental Sciences, Kerela, India.
4 Senior Lecturer, Department of Oral and Maxillofacial Pathology, K.S.R. Institute of Dental Science and Research, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Yoithapprabhunath Thukanayakanpalayam Ragunathan, Reader, Department of Oral and Maxillofacial Pathology, Vivekanandha Dental College for Women, Elayampalayam, Thiruchengodu, Namakkal (Dist), Tamilnadu-637205, India.
E-mail: yoitha.dentist@gmail.com
Introduction
Ameloblastoma holds a unique position among benign tumours by its locally destructive and invasive nature. Recently improvised molecular techniques helped researchers to unravel the myth behind such biologic behaviour. Though interesting findings have been delivered, the rhythmic correlation regarding the exact mechanism still remains lacking. Neurotrophins and their receptor mediated pathways play a crucial role in survival, death and differentiation of many neuroectoderm derived cells. With this background, the study has been aimed to investigate the expression of p75-NTR (Neurotrophin Receptor) in follicular and plexiform ameloblastoma.
Aim
To analyze the immunohistochemical expression pattern of p75-NTR in ameloblastoma and to compare the immunohistochemical expression pattern of p75-NTR among the histological types of ameloblastoma, follicular and plexiform patterns.
Materials and Methods
Total 22 ameloblastomas (12 follicular, 10 plexiform) were immuno-stained with anti-human p75-NTR mouse IgG monoclonal antibody and the pattern of staining is statistically analyzed.
Results
Only 11 (10 follicular, 1 plexiform) out of 22 ameloblastomas showed immuno-reactivity to p75-NTR. In ameloblastoma, only the peripheral pre-ameloblast like tall columnar cells showed reactivity whereas the stellate reticulum-like cells were immuno-negative. The staining pattern was membranous in the immuno-reactive cells. The results were studied with the downstream pathways from the literature and a possible mechanism has been proposed.
Conclusion
The expression pattern of p75-NTR was found to be more in follicular ameloblastoma than plexiform.
Ameloblastoma, Odontogenic tumor, Immunohistochemistry, Regulation, p75-Neurotrophin receptor (p75NTR)
Introduction
Ameloblastoma, a neoplasm derived from odontogenic epithelium holds a unique position among benign tumours by its locally destructive and invasive nature, with high propensity for recurrence. It is believed to arise from the remnants of odontogenic epithelium, epithelial lining of odontogenic cysts and basal layer of overlying mucosa. It is the second most common odontogenic neoplasm next to odontoma in all ethnic groups, representing about 1% of head and neck neoplasm which does not undergo differentiation to enamel formation. These tumours have tendency to recur [1]. Robinson described ameloblastoma as a benign tumour that is usually “unicentric, nonfunctional, intermittent in growth, anatomically benign and clinically persistent” [2]. Ameloblastoma cells have various proliferating activities depending on the histological type and cytological pattern. Both apoptosis and proliferating activity of the cell are implicated in the development of ameloblastoma. The peripheral anti-apoptotic proliferating site and the inner central proapoptotic differentiating site are the two relatively distinct patterns and key features regarding ameloblastoma. The peripheral cells of the tumour which have chosen the cell survival mode is considered as the reason behind the progression of the disease. The knowledge about this ameloblastoma is gaining greater importance because of its emerging variants [3]. Though ameloblastoma is benign, the aggressive nature is recognized by its invasion into surrounding bone via osteoclastic bone destruction [4].
Improvised molecular techniques in the past few decades have helped the researchers to unravel the myth behind such biologic behaviour [4]. Clonality, cell cycle regulation, role of apoptosis and tumour suppressor genes, enamel matrix proteins, bone resorbing and stromal invasion mechanism and other signalling molecules in relation to the pathogenesis of ameloblastoma have been studied to demonstrate the biological behaviour of this tumour [5]. Monoclonal pattern was seen in solid/multicystic ameloblastoma and it supports the hypothesis that an initial mutation is the first event in the development of the tumour [6]. The ability of local invasion and high recurrence rate of this tumour is confirmed by the telomerase activity seen in ameloblastoma [7]. The functional immature state of the ameloblastoma cell lines when comparing with the enamel matrix producing ameloblast cells is due to the unexpressed enamel forming proteins [8].
Human p75 Neurotrophin Receptor (p75-NTR), is also known as Nerve Growth Factor Receptor (NGFR) and it is a member of the TNF receptor super family. It maps to 17q21 and encodes a 75 kDa cell surface receptor glycoprotein [9]. Formerly it was believed that, p75 has selective affinity towards nerve growth factor receptor (LNGFR), but latter it was found out that p75 has high affinity towards all four types of neurotrophins similar to Tyrosin Kinase Receptor (Trk) and hence the name p75-NTR. It is also called as Tumour Necrosis Factor Receptor II (TNFR-II). Neurotrophins and their receptor mediated pathways play a decisive role in the death and survival of many neuroectoderm derived cells [10–12]. Apart from these roles, numerous studies indicate that p75-NTR may also support cellular differentiation, growth, migration and also plays a role in the down regulation of NF-κB signal transduction pathway [13–15].
Spatiotemporal expression of p75-NTR during pre-natal human tooth development is specifically detected in the tooth buds and dental follicle. Prior to matrix deposition, the entire inner enamel epithelium and dental follicle display immunoreactivity and the immunoreactivity is lost after matrix deposition is initiated [16]. The p75-NTR may be involved in differentiation and/or proliferation of human odontogenesis [17]. Peripheral cells of ameloblastoma resemble pre-secretory ameloblast cells of odontogenesis.
Till date traditional chemotherapy and radiation has not been explored or contraindicated in ameloblastoma and surgery is regarded as the only treatment of choice. The molecular pathogenesis behind ameloblastoma tumour has never attained totality, because of the lack in rhythmic correlation regarding the exact mechanism. By obtaining thorough knowledge on altered molecular signaling pathways in this tumour will definitely reveal the mechanisms of tumourigenesis, differentiation, proliferation and tumour progression which in turn may bring us to the brink of nonsurgical treatment for ameloblastoma [18].
With this background, the immunohistochemical study was aimed to analyze the immunohistochemical expression pattern of p75NTR in ameloblastoma and to compare the immunohistochemical expression pattern of p75NTR among follicular and plexiform types of ameloblastoma.
Materials and Methods
This laboratory study comprised of 22 samples comprising 12 follicular and 10 plexiform variants of ameloblastoma that were subjected to histological examination in the Department of Oral and Maxillofacial Pathology, Rajah Muthiah Dental College & Hospital, Annamalai University, India. These archival tissues were fixed in 10% buffered formalin and embedded in paraffin wax, two sections of 3 to 4 μm thickness were prepared in Rotary Microtome (LEICA RM 2125RT, Germany) from each case for doing routine hematoxylin and eosin stain and immunohistochemical staining. For each specimen, one slide was stained with haematoxylin and eosin to confirm the diagnosis and another was used for immunohistochemical analysis. In all cases, under the hematoxylin and eosin staining, morphological analysis disclosed the presence of more than one histological pattern of ameloblastoma and the pattern which predominated was considered for final diagnosis. All the 22 cases were subjected to immunohistochemistry to evaluate the expression of p75-NTR. The sections were deparaffinised by heating on the slide warmer at 60°C for one and half hours. The sections were dewaxed in two changes of xylene, each for 15 minutes. The sections were rehydrated in graded alcohol (100%, 90%, 70%, and 50%), 5 minutes each and kept under water for 10 minutes. The slides were placed in a coupling jar, with citrate buffer solution which in turn was kept in a pressure cooker containing water. The pressure cooker was then closed with the lid and brought to full pressure. Timing was noted down after the full pressure was reached and was kept for 10 minutes duration. The pressure cooker was allowed to cool down to room temperature before removal of slides. The advantages of pressure cooker antigen retrieval method were, even distribution of heat over the slides, minimal reagent evaporation, excellent heat-source regulation with temperature range between - 25°C to 125°C. All the reagents stored in the refrigerator were brought to room temperature prior to immunostaining. All the incubations were performed at room temperature using a humidifying chamber. At no time the tissue sections were allowed to dry during the staining procedure.
After tapping off the excess buffer from the slide, the sections were covered with 3% hydrogen peroxide for 10 minutes, following which it was treated with power block (DAKO REAL En Vision, Denmark) for 10 minutes to avoid cross reactions and to reduce non-specific binding which was then gently washed with phosphate wash buffer (pH: 7.4). Excess buffer was tapped off and the sections were covered completely with optimally diluted mouse monoclonal anti-p75-NTR antibody (Santa Cruz Biotechnology, Inc. USA) in 1:50 dilutions in Phosphate Buffered Saline (PBS) for one and half hours. Then the slides were washed gently with PBS and kept in the PBS buffer bath for 10 minutes. The slides were washed and treated with secondary antibody tagged with Poly Horseradish Peroxidase enzyme (HRP) (DAKO REAL En Vision, Denmark) for 30 min. The slides were then washed with PBS and immunostaining was developed by treatment with freshly prepared DAB (3, 3-diaminobenzidene tetra hydrochloride) solution for 5 minutes following which it was washed in distilled water to remove excess chromogen. The slides were immersed in Mayer’s hematoxylin for 7 minutes, and blueing was done in running tap water for 10 minutes. The sections were dehydrated in graded alcohol (50%, 70%, 90%, and 100%); air dried for 10 minutes, cleared with xylene and mounted using DPX, a non-aqueous permanent mounting medium.
Interpretation of staining: Presence of brown colour end product at the site of target antigen was considered as positive immunoreactivity. The immunostained slides were observed for positivity under 100x/400x magnifications and recorded with a high quality photomicrograph. The staining was scored by evaluating the positive and negative immunoreactivity of each slide. The scores for positive immunoreactivity were given as ‘1’ and for negative immunoreactivity as ‘0’.
Statistical Analysis
Statistical package for social science (SPSS) software version 14, 2008 was used for statistical analysis. The level of significance (p<0.05) was employed in all statistical comparisons. Quantitative data were recorded as mean ± standard deviation. The expressions of p75-NTR between follicular and plexiform type of ameloblastoma were analyzed statistically using Chi-square test.
Results
Total 22 samples of central ameloblastoma in the study group were taken for assessment. Out of which 12 were follicular type and remaining were plexiform type. Among 12 samples of follicular type 10 (83.3%) samples showed positive immunoreactivity [Table/Fig-1] and remaining samples were negative. Among 10 plexiform samples, one (10%) sample showed positive for immunostaining and remaining samples were negative [Table/Fig-2]. The expression of p75NTR was compared between the follicular ameloblastoma and plexiform ameloblastoma statistically by using Chi-square test. The results were statistically significant between these two groups at p value<0.05. The comparative result between follicular and plexiform ameloblastoma is given in [Table/Fig-3].
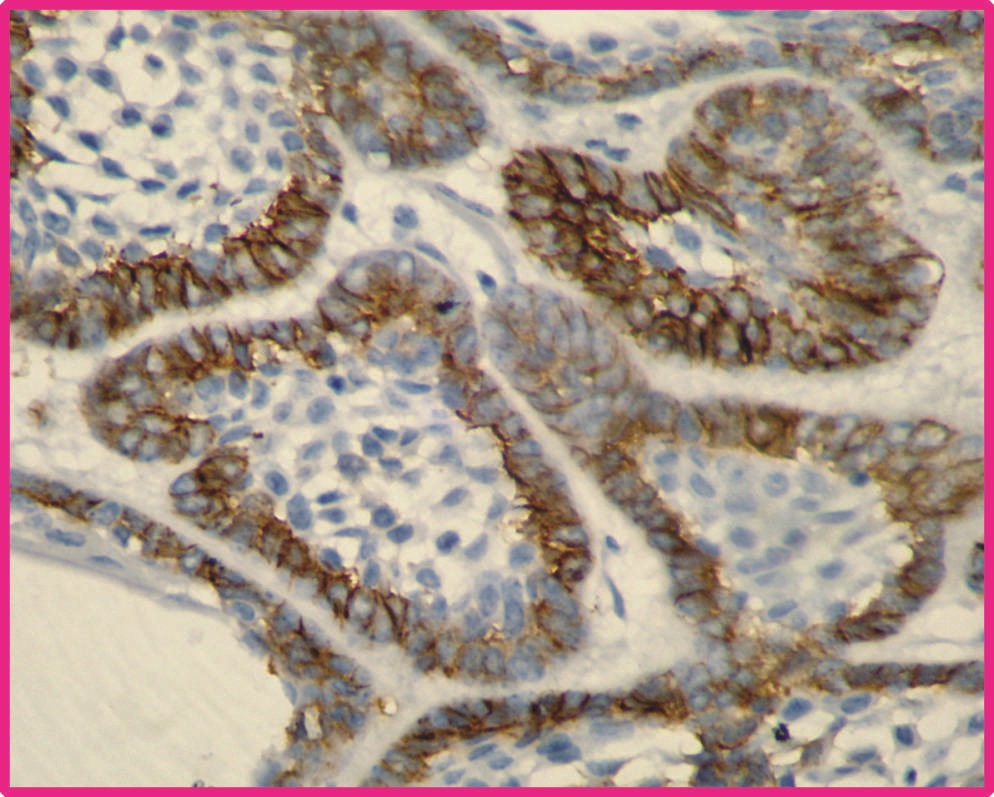
Follicular ameloblastoma showing positive p75-NTR immunoexpression in peripheral cells (Photomicrograph 40X).
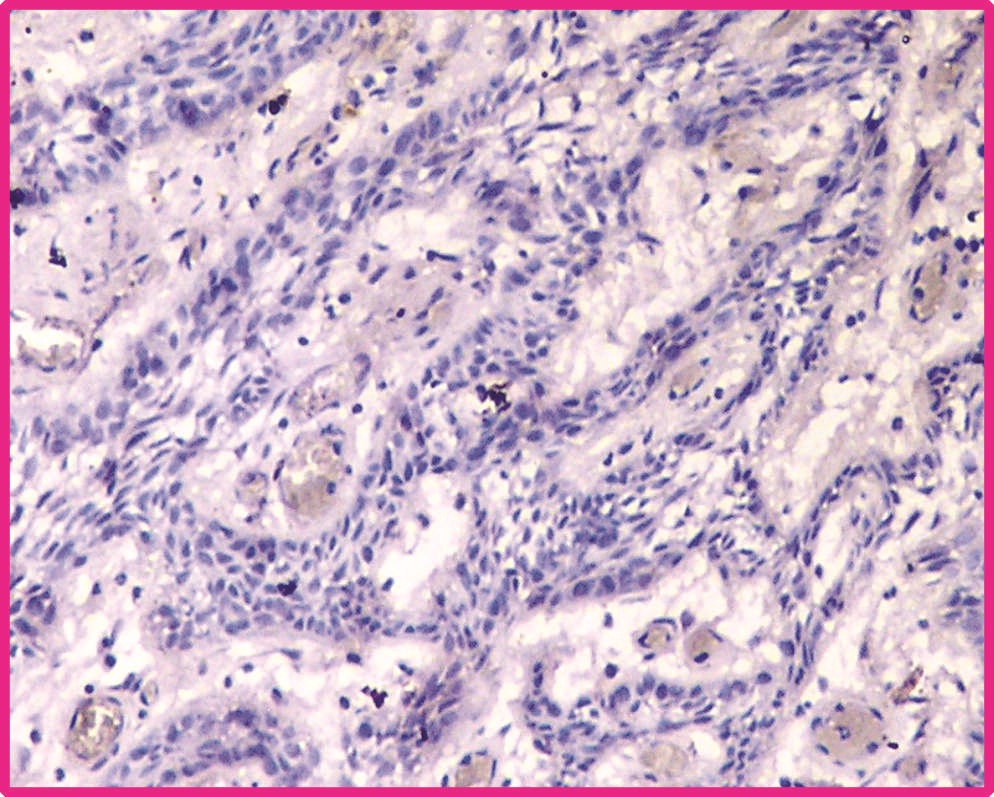
Plexiform ameloblastoma showing negative p75-NTR immuno-expression in peripheral cells (Photomicrograph 40X).
Comparison of two histological growth pattern of follicular and plexiform ameloblastoma with respect to labelling index of p75-NTR by Chi-square test.
| Groups | Totalnumber ofcases | Number ofpositivecases (%) | Numberof negativecases (%) | Chi-squarevalue | p-value |
|---|
| Follicular ameloblastoma | 12 | 10(83.3) | 2(16.7) | 11.7 | 0.001Significant |
| Plexiform ameloblastoma | 10 | 1(10) | 9(90) |
Discussion
Ameloblastoma is the most frequently encountered tumour that stems from the odontogenic ectoderm. Ameloblastoma is histologically benign, locally aggressive and invasive, usually grows and progress slowly and has high tendency to recur. The progression of the tumour can result in severe deformities of the craniofacial complex. A few cases of tumour-to-tumour metastasis as well as malignant transformation with distant metastasis have been reported [19]. Tumor-to-tumor metastasis criteria includes: a) the metastatic focus must be at least partially enclosed by a rim of benign, histologically distinct host tumor tissue; and b) the existence of a primary carcinoma must be proven and be compatible with the metastasis [20]. Genetic and cytogenetic alterations in their structures were detected by several advanced investigations. However complete ravelling on the oncogenesis, cytodifferentiation and tumour progression has not been done [4].
During odontogenesis, before matrix deposition, the entire inner enamel epithelium along with dental follicle display immunoreactivity for p75-NTR, but immunoreactivity is lost after inner enamel epithelium is differentiated into ameloblast and initiate matrix deposition [16,17]. Similar types of cells (ameloblast like cells) were found in the peripheral layers of ameloblastoma. Though they resemble ameloblast cells, matrix production is absent due to the lack of ameloblastin, enamelin and sheathlin proteins [8]. This suggests that the tumour cells do not attain functional maturation like secretory phase ameloblasts. In regard to this evidence, the present study was carried out to check for the p75-NTR expression and the possible role of p75-NTR in the proliferating front of ameloblast like cells present in the ameloblastoma.
In present study, p75-NTR immunoexpression was positive in peripheral cells of follicular ameloblastoma cases (83.3%) comparing to that of plexiform ameloblastoma cases (10%). This reveals that there is a significant difference in the expression of p75-NTR between follicular ameloblastoma and plexiform ameloblastoma cases. The positive immunoexpression of p75-NTR was found in 50% of the ameloblastoma cases. The expression in ameloblastoma cases is restricted only to the cell membrane of cuboidal or columnar type of peripheral cells of the tumour islands and cords. None of the central stellate-like cells or stromal components stained positive for p75-NTR.
For any tumour growth, cell proliferation and matrix degradation plays an essential role. Ameloblastoma has two relatively different patterns, showing outer layer of anti-apoptotic proliferation layer and central pro-apoptotic differentiation area. In literature, studies showing the expression of anti-apoptotic proteins bcl-2 and bcl-X were confined to the peripheral basal cell layer of the ameloblastoma tumour island concluding that ameloblastoma has much more apoptosis-inhibiting protein than the apoptosis-modulating protein [21]. Immunohistochemical study on survivin expression in ameloblastoma, showed immunoexpression confined to peripheral layers of ameloblastoma and hence suggested that the ameloblastoma might have a high survival activity and this might be one of the reasons behind the high recurrence rate [22].
Proliferating Cell Nuclear Antigen (PCNA) is a co-factor of DNA polymerase δ, which participates in the replication of the leading and lagging chains of DNA and in repair of damaged DNA. Expression of PCNA in ameloblastomas and odontogenic cysts study showed high labelling of proliferative index expression in the peripheral ameloblast like cells [23]. Cyclin D1, an essential molecule for the dividing cell to enter into DNA synthesis phase, controls the cell cycle transit from the G1 to S phase [24]. According to a study, there was immunoexpression of Cyclin D1 in both peripheral and central cells of ameloblastoma. However, there was a difference in the proliferating behaviour between the peripheral cells of follicular and plexiform patterns [25].
Nuclear Factor κB (NF-κB) is a ubiquitously expressed transcription factor that plays a critical role in the expression of various inducible target genes that control apoptosis among several other vital functions [26]. The immunoexpression of NF-κB proved to be positive in ameloblastoma both in central and peripheral cells, irrespective of its histological types [27]. NF-κB activates distinct prosurvival bcl-2 family proteins and suggests a role for these factors in the inhibition of cell death by Rel/NF-κB [28]. Akt signaling pathway was the downstream signal of NF-κB, which means regulation of such signal is due to the regulation of NF-κB [29]. Strong immunoreactivity for Akt in peripheral columnar cells and weak reactivity in central polyhedral cells were identified in plexiform and follicular ameloblastoma through immunohistochemical study [30]. Matrix metalloproteinases are an important group of zinc enzymes responsible for degradation of the extracellular matrix components such as collagen and proteoglycans in normal embryogenesis and remodelling and in many disease processes especially for tumour progression [31]. These molecules play a significant role in tumour invasion and progression. Strong expression of MMP-2, MMP-9 and TIMP-1 was found in stromal cells of both follicular and plexiform type [32].
In literature, p75-NTR expression also regulates the cyclin D1 along with cyclin A, cyclin E, and cyclin dependent kinases like cdk 2. Up regulation of p75-NTR in human hepatocellular carcinoma, down regulated the expression of PCNA [33]. The p75-NTR could suppress NF-κB through IκB kinase (IKKs). Expression of p75-NTR in human gastric cancer cells had a function of inhibiting the invasive and metastatic abilities in vivo & in vitro, which was mediated, at least partially by, down-regulation of matrix metalloproteinase MMP-9, and up regulation of tissue inhibitor of matrix metalloproteinase TIMP-1 proteins, via the NF-κB signal transduction pathway [15, 34].
Till date, there is no clinical relevance between the histological variant of follicular and plexiform ameloblastoma [35]. Significant expression of p75-NTR in peripheral ameloblast like cells of follicular ameloblastoma was appreciated in the present study. Binding of neurotrophins with p75-NTR induces Trk dependent or Trk independent receptor-mediated pathways that results in apoptosis, survival, differentiation, growth and migration of many neuroectoderm derived cells. It also plays a role in the down regulation of NF-κB signal transduction pathway [10–15]. The activation, pathway and role of p75-NTR in ameloblastoma have not been studied before. The association between p75-NTR and its downstream molecules in ameloblastoma was not made through previous studies. This limits the present study in finding out the complete detail of p75-NTR role in follicular and plexiform ameloblastoma. But by analyzing the role of p75-NTR in other tumours through the course of literature, the expression of p75-NTR regulates cell cycle regulators (Cyclins), anti apoptotic proteins (bcl-2, bcl-X), cell proliferation enzymes (PCNA), and tumour progression molecules (MMP-9 and TIMP-1) through NF-κB which could be the reason behind the differed biological behaviour between follicular and plexiform ameloblastoma.
Limitation
In future, increasing the sample size and finding the link between p75-NTR and its downstream molecules in ameloblastoma will bring out more facts regarding the biological behaviour.
Conclusion
The positive expression of p75-NTR was found only in peripheral cells that resembled pre-ameloblasts in most of the follicular ameloblastoma. The positive expression of p75-NTR may have a responsibility in regulating cyclin D1, PCNA, bcl-2, bcl-X, NF-κB, MMP-9 and TIMP-1. Expression of p75-NTR could be the possible reason behind the differed biological behaviour between follicular and plexiform ameloblastoma. Further studies focusing on the possible pathway connecting p75-NTR and other prognostic molecular markers of ameloblastoma will unravel the ground-breaking function played by it. Using the key words: p75-NTR, NGFR, ameloblastoma (Follicular, Plexiform) in Google and Pubmed search, no study was available that showed the immunohistochemical analysis of p75-NTR in ameloblastoma tumours. To the best of our knowledge, this was the very first study made to analyse the immunoexpression and to find the possible role of p75-NTR in ameloblastoma.
[1]. Reichart PA, Philipsen HP, In: Reichart PA, Philipsen HP, editors. Odontogenic tumors and allied lesions. Chapter 5- Solid/Multicystic Ameloblastoma 2004 LondonQuintessence Publishing Co Ltd:43-58. [Google Scholar]
[2]. Shafer Hine Levy In: Rajendran R, Sivapathasundharam B, editors. Shafer’s Text book of Oral pathology. Chapter I (4)- Cysts and tumors of odontogenic origin 2009 7th edNew DelhiElsevier publications:276 [Google Scholar]
[3]. Sandra F, Nakamura N, Mitsuyasu T, Two relatively distinct patterns of ameloblastoma: an anti-apoptotic proliferating site in the outer layer (periphery) and a pro-apoptotic differentiating site in the inner layer (centre)Histopathol 2001 39:93-98. [Google Scholar]
[4]. Stolf DP, Karim AC, Banerjee AG, Genetic aspect of ameloblastoma: a brief reviewBiotechnol Mol Biol Rev 2007 2:116-22. [Google Scholar]
[5]. Gomes CC, Duarte AP, Gonc M, Diniz A, Current concepts of ameloblastoma pathogenesisJ Oral Pathol Med 2010 39:585-91. [Google Scholar]
[6]. Gomes CC, Oliveira CS, Castro WH, Clonal nature of odontogenic tumoursJ Oral Pathol Med 2009 38:397-400. [Google Scholar]
[7]. Kumamoto H, Kinouchi Y, Ooya K, Telomerase activity and telomerase reverse transcriptase (TERT) expression in ameloblastomasJ Oral Pathol Med 2001 30:231-36. [Google Scholar]
[8]. Saku T, Okabe H, Shimokawa H, Immunohistochemical demonstration of enamel proteins in odontogenic tumoursJ Oral Pathol Med 1992 21:113-19. [Google Scholar]
[9]. Reis-Filho JS, Steele D, Palma S, Jones RL, Savage K, James M, Distribution and significance of nerve growth factor receptor (NGFR/p75NTR) in normal, benign and malignant breast tissueMod Pathol 2006 19:307-19. [Google Scholar]
[10]. Huebner K, Isobe M, Chao M, The nerve growth factor receptor gene is at human chromosome region 17ql2-17q22, distal to the chromosome 17 breakpoint in acute leukemiasProc Natl Acad Sci USA 1986 83:1403-07. [Google Scholar]
[11]. Roux PP, Barker PA, Neurotrophin signaling through the p75 neurotrophin receptorProg Neurobiol 2002 67:203-33. [Google Scholar]
[12]. Nykjaer A, Willnowand TE, Petersen CM, p75NTR – live or let dieCurr Opin Neurobiol 2005 15:49-57. [Google Scholar]
[13]. Yamashita T, Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowthNeuron 1999 24(3):585-93. [Google Scholar]
[14]. Anton ES, Weskamp G, Reichart LF, Matthew WD, Nerve growth factor and its low-affinity receptor promote Schwann cell migrationProc Natl Acad Sci USA 1994 91(7):2795-99. [Google Scholar]
[15]. Jin H, Pan Y, Zhao L, Zhai H, p75 Neurotrophin receptor suppresses the proliferation of human gastric cancer cellsNeoplasia 2007 9:471-78. [Google Scholar]
[16]. Becktor KB, Hansen BF, Nolting D, Kjaer I, Spatiotemporal expression of NGFR during pre-natal human tooth developmentOrthod Craniofacial Res 2002 5:85-89. [Google Scholar]
[17]. Christensen LR, Mollgerd K, Kjaer I, Janas MS, Immunocytochemical demonstration of nerve growth factor (NGF-R) in developing human fetal teethAnat Embryol 1993 188:247-55. [Google Scholar]
[18]. Sauk JJ, Nikitakis NG, Are we on the brink of nonsurgical treatment for ameloblastoma?Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010 110:68-75. [Google Scholar]
[19]. Huang IY, Lai ST, Chen CH, Chen CM, Wu CW, Shen YH, Surgical management of ameloblastoma in childrenOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 104(4):478-85. [Google Scholar]
[20]. Moody P, Murtagh K, Piduru S, Tumor-to-tumor metastasis: pathology and neuroimaging considerationsInt J Clin Exp Pathol 2012 5(4):367-73. [Google Scholar]
[21]. Luo HY, Yu SF, Li TJ, Differential expression of apoptosis-related proteins in various cellular components of ameloblastomasInt J Oral Maxillofac Surg 2006 35:750-55. [Google Scholar]
[22]. Kumamoto H, Ooya K, Expression of survivin and X-chromosome-linked inhibitor of apoptosis proteinVirchows Arc 2004 444:164-70. [Google Scholar]
[23]. Fioroni PM, Santinelli A, Rubini C, Expression of proliferating cell nuclear antigen in ameloblastomas and odontogenic cystsOral Oncol 1998 34:408-12. [Google Scholar]
[24]. Baldin V, Lukas J, Marcote MJ, Cyclin D1 is a nuclear protein required for cell cycle progression in G1Genes Dev 1993 7:812-21. [Google Scholar]
[25]. Kumar H, Vandana R, Kumar GS, Immunohistochemical expression of cyclin D1 in ameloblastoma and adenomatoid odontogenic tumoursJ Oral Maxillofac Pathol 2011 15:283-87. [Google Scholar]
[26]. Guttridge DC, Albanes C, Reuther JY, NF-κB controls cell growth and differentiation through transcriptional regulation of Cyclin D1Mol Cell Biol 1999 19(8):5785-99. [Google Scholar]
[27]. Hendarmin L, Kawano S, Yoshiga D, An anti-apoptotic role of NF-κB in TNFα induced apoptosis in an ameloblastoma cell lineOral Sci Inter 2008 5(2):96-103. [Google Scholar]
[28]. Chen C, Edelstein LC, Gélinas C, The Rel/NF-kB family directly activates expression of the apoptosis inhibitor Bcl-xLMol Cell Biol 2000 20(8):2687-95. [Google Scholar]
[29]. Meng F, Liu L, Chin PC, Mello SR, Akt Is a downstream target of NF-κBJ Biol Chem 2002 277(33):29674-80. [Google Scholar]
[30]. Kumamoto H, Ooya K, Immunohistochemical detection of phosphorylated Akt, PI3K, and PTEN in ameloblastic tumorsOral Dis 2007 13:461-67. [Google Scholar]
[31]. Woessner JF, Matrix metalloproteinases and their inhibitors in connective tissue remodelingFASEB J 1991 5:2145-54. [Google Scholar]
[32]. Kumamoto H, Yamauchi K, Yoshida M, Ooya K, Immunohistochemical detection of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in ameloblastomasJ Oral Pathol Med 2003 32:114-20. [Google Scholar]
[33]. Yuanlong H, Haifeng J, Xiaoyin Z, The inhibitory effect of p75 neurotrophin receptor on growth of human hepatocellular carcinoma cellsCancer Lett 2008 268:110-19. [Google Scholar]
[34]. Jin H, Pan Y, He L, p75 Neurotrophin receptor inhibits invasion and metastasis of gastric cancerCancer Res 2007 5:423-33. [Google Scholar]
[35]. Gardner DG, Heikinheimo K, Shear M, Philipsen HP, Coleman H, In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of TumoursPathology and Genetics of Head and Neck Tumours. Chapter 6-Odontogenic tumours 2005 LyonIARC Press:296-300. [Google Scholar]