Introduction
Cyclosporine, an immunosuppressive agent used in the management of renal transplant patients is known to produce Drug Induced Gingival Overgrowth (DIGO) as a side effect. Several mechanisms have been elucidated to understand the pathogenesis of DIGO. Recently, epithelial mesenchymal transition has been proposed as a mechanism underlying fibrosis of various organs.
Aim
The aim of the study was to investigate if Epithelial Mesenchymal Transition (EMT) operates in Cyclosporine induced gingival overgrowth.
Materials and Methods
The study involved obtaining gingival tissue samples from healthy individuals (n=17) and subjects who exhibited cyclosporine induced gingival overgrowth (n=18). Presence and distribution of E-Cadherin, S100 A4 and alpha smooth muscle actin (α-SMA) was assessed using immunohistochemistry and cell types involved in their expression were determined. The number of α– SMA positive fibroblasts were counted in the samples.
Results
In control group, there was no loss of E-Cadherin and a pronounced staining was seen in the all layers of the epithelium in all the samples analysed (100%). S100 A4 staining was noted in langerhans cells, fibroblasts, endothelial cells and endothelial lined blood capillaries in Connective Tissue (CT) of all the samples (100%) while α - SMA staining was seen only on the endothelial lined blood capillaries in all the samples (100%). However in DIGO, there was positive staining of E-Cadherin only in the basal and suprabasal layers of the epithelium in all the samples (100%). Moreover there was focal loss of E-Cadherin in the epithelium in eight out of 18 samples (44%). A break in the continuity of the basement membrane was noted in three out of 18 samples (16%) on H & E staining.
Conclusion
Based on the analysis of differential staining of the markers, it can be concluded that EMT could be one of the mechanistic pathways underlying the pathogenesis of DIGO.
Introduction
Despite advances in the field of drug discovery, no drug is free of side effects. The use of certain medically important pharmacological agents by practitioners has been quite inevitable. Cyclosporine (immunosuppressant), nifedipine (antihypertensive) and phenytoin (antiepileptic) are drugs that have been trusted and widely used in medicines. Even though newer drugs like tacrolimus with lesser side effects have been used now for immunosuppression, the administration of cyclosporine has not been withdrawn completely. The intraoral fibrosis manifests in the gingiva and is known as Drug Induced Gingival Overgrowth (DIGO) as it is associated with drug usage. DIGO was previously called gingival hyperplasia or hypertrophy. It was Hassell who coined the term DIGO as this condition and is characterized by increase in number of cells and matrix content, however the ratio of cell to matrix remains unchanged; hence the term overgrowth or increased growth [1]. This disease manifests as hyperplasia of the junctional epithelium, hypertrophy of the keratinized epithelium and excessive Connective Tissue (CT) accumulation in the gingiva as documented by Ayanoglou & Lesty [2]. The histological characteristics of DIGO include increase in thickness of the epithelium and elongation of rete pegs along with an increase in the number of fibroblasts and blood vessels in the CT matrix with an increase in ground substance as observed by Wondimu B [3].
Although the pathogenesis of DIGO is not definitively known, this disorder seems to be induced through the disruption of homeostasis of collagen synthesis and degradation of the gingival CT matrix resulting in the excessive accumulation of collagen fibers. Investigations of the non-collagenous matrix have also revealed that the glycosaminoglycans and proteoglycans comprise 20% of the dry weight in gingival tissues of DIGO and are significantly increased in quantity as compared to only 7% in normal tissue [4]. They reported that the variation in the amount of collagen and non-collagenous proteins could be attributed to increased proliferation, delayed apoptosis or prolonged survival of the fibroblasts which are the principal synthetic cells of the gingival connective tissue. An over expression of bcl-2 and c-Myc in gingival overgrowth induced by nifedipine and phenytoin has been demonstrated, suggesting their role in the pathogenesis of DIGO [5]. On the other hand a study by Saito K [6] and co-workers has also shown over expression of Ki-67, a proliferation associated marker in gingival fibroblasts in patients with Phenytoin and Nifedipine induce gingival overgrowth. It can hence be hypothesized that the fibroblasts either demonstrate increased proliferation or reduced apoptosis which enhances their survival and synthetic activity in DIGO.
With regard to the origin of these cells in DIGO a working model that could be proposed is that, fibroblasts may be generated from a variety of sources like fibrocytes [7], haematopoietic stem cells [8] as well as from epithelial and endothelial cells through a process termed Epithelial/Endothelial Mesenchymal (EMT/ End MT) transition [9]. Epithelial Mesenchymal Transition (EMT) is a process in which epithelial cells migrate into the CT and trans-differentiate into fibroblast-like cells; this occurs as the epithelial cell–cell and cell-extracellular matrix interactions are destabilized [10]. EMT is considered as a normal phenomenon in developmental stages of embryo (Type I EMT), in mature tissues it is considered to occur due to a pathological process as seen in progression of tumors of epithelial origin (Type III EMT) and heart, lung, liver & kidney fibrosis (Type II EMT) [11]. In this regard E-Cadherin is considered as a prototypical epithelial marker of EMT. The reduction in epithelial expression of E-Cadherin also called the Cadherin switch has been known to promote EMT by facilitating weakening of the intercellular junctions and promoting movement of epithelial cells towards the connective tissue [12]. S100 A4 also called fibroblast specific protein is a calcium binding protein that is regarded a prototypical fibroblast marker in EMT associated with cancer and fibrosis [13]. Alpha smooth muscle positive myofibroblasts have been demonstrated in type 2 EMT [14]. In the process of analysing if EMT contributes to the pathogenesis of Cyclosporine induced gingival overgrowth, we performed this study to assess the expression of EMT markers such as E-Cadherin, S100A4 and alpha smooth muscle actin (α-SMA) in gingival tissue samples.
Materials and Methods
A total of 35 subjects were recruited for the case control study from the Outpatient Department of Periodontology, Faculty of Dental Sciences and from the Department Of Nephrology, Sri Ramachandra University, Chennai, India. The sample size was determined based on the prevalence of cyclosporine induced DIGO [15]. The study was approved by the Institutional Ethics Committee of Sri Ramachandra University and the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975 that was revised in 2000. The protocol was explained and a prior informed consent was obtained from all the subjects. The selected subjects were divided into two groups, healthy individuals who exhibited clinically healthy gingiva (control; n=17) and subjects with Cyclosporine induced gingival overgrowth (test; n=18).
Periodontal examination: Individuals free of systemic disease having clinically healthy periodontium as evidenced by absence of bleeding on probing, with no attachment loss, radiographic evidence of bone loss or any previous history of periodontal disease were categorized into the control group. Patients who were on cyclosporine therapy following renal transplant for a minimum duration of six months manifesting clinical signs of gingival overgrowth at least in one site according to the overgrowth severity index by Angelopoulos & Goaz [16] were enrolled in the test group. The exclusion criteria for both the groups were subjects who smoked/used smokeless tobacco/consumed alcohol and consumed anti-inflammatory drugs in the past six months. Pregnant and lactating women were also excluded from the study. Patients who consumed drugs other than Cyclosporine that could cause gingival overgrowth were also excluded from the study.
Gingival tissue sample collection: Gingival tissue samples were collected from healthy individuals (control group) undergoing crown lengthening procedures and therapeutic orthodontic extractions. A part of the papilla either mesial or distal to the tooth to be removed was excised with a surgical blade under local anaesthesia prior to the extraction procedure under aseptic precautions. DIGO samples were obtained during gingivectomy procedure from sites that demonstrated overgrowth severity score of 2. The excised tissues were washed in saline and fixed in 10% buffered formalin solution. The tissues were transferred to the pathology laboratory for paraffin embedding. From each block four serial sections were prepared on poly l-lysine coated slides, one section for Haematoxylin & Eosin (H&E) staining and the other three were used for immunohistochemistry to detect E-Cadherin, S100A4 & α-SMA.
Immunohistochemistry: Immunohistochemical staining was accomplished using mouse monoclonal primary antibodies against human E-Cadherin, human S100 A4 & human α-SMA and a universal secondary antibody kit (super sensitive polymer HRP IHC detection system)(Biogenex, The Netherlands). The formalin fixed and paraffin embedded tissue sections of 5μm thickness were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide and non-specific binding was blocked with a universal blocking agent. The tissue sections were washed in phosphate buffered saline solution. All the three primary antibodies were used at a dilution of 1:100. Incubation time for the primary antibodies was one hour and for the secondary antibody of 1:100 dilution was 30 minutes at room temperature. A Strep AB Complex/ HRP was applied and the specimens were stained with diaminobenzidine and counterstained with Mayers Haematoxylin. Positive controls for E-Cadherin, S100A4 & α-SMA were colon carcinoma, neurofibroma and leiomyosarcoma respectively. Negative controls consisted of substitution of primary antibody with rabbit/mouse serum. After completion of the IHC procedures, the slides were observed by a trained pathologist who graded the staining intensity according to the criteria proposed by Carrillo et al., [17]. The number of α-SMA positive fibroblasts per field of view were counted at 40X magnification and documented. The specimens showing the largest and smallest number of CD1a-positive cells were used as reference and categorised as presenting intense (3) and discrete (1) immunostaining, respectively. The other specimens were categorised as presenting moderate (2) or none (0) immunostaining.
Results
Clinical and Histopathologic asssesment: The demographic data of the study participants is shown in [Table/Fig-1]. Gingival bleeding on probing and other clinical signs of inflammation were absent in sites chosen for sampling healthy gingival tissues. All DIGO sites chosen for gingivectomy exhibited overgrowth severity value of 2 according to the index proposed by Angelopoulos & Goaz [16]. On H&E staining, basement membrane break was seen in three out of 18 samples analysed (16%).
Demographic data of the study population.
| Variables | Healthy Individuals (Control) (n=17) | Cyclosporine induced gingival overgrowth (DIGO) (n=18) |
|---|
| Mean Age (years) | 48.3±3.5 | 46.2±2.6 |
| Gender (M/F) | 10/7 | 10/8 |
| Duration of Cyclosporine use (years) | - | 6±3 |
| Plaque Index (%) | 19±14 | 67±34 |
| Gingival Bleeding score (%) | 5±2 | 65±24 |
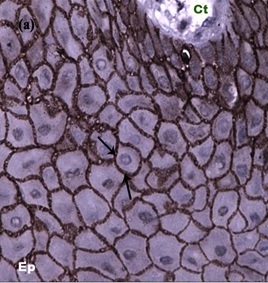
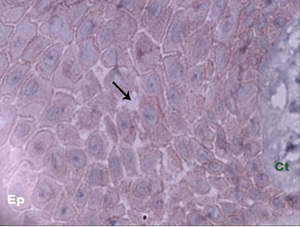
Immunohistochemical analysis: [Table/Fig-2] shows the pattern of distribution of E-Cadherin in the samples analysed. All the 17 healthy samples (100%) expressed moderate to intense staining in the all layers of the epithelium especially at the intercellular junctions [Table/Fig-3]. However, all the 18 DIGO samples expressed weak staining in the basal and suprabasal layers of the epithelium (100%). Focal loss of expression of E-Cadherin was seen in eight out of 18 samples (44%). Weak staining was observed in the intercellular areas showing oedema [Table/Fig-4]. Fragmented expression and loss of expression was seen in the basal layer.
Staining pattern and distribution of E-Cadherin in Group -I and Group -II.
| Group | E-Cadherin staining intensity and distribution | No. of samples reported positive | % of samples reported positive |
|---|
| Healthy individuals (I) | Moderate to intense staining in all layers of epithelium in intercellular junctions | 17/17 | 100% |
| DIGO (II) | Weak staining in basal and supra basal layers of epithelium | 18/18 | 100% |
| Focal loss of E-Cadherin expression | 8/18 | 44% |
| Loss of expression in basal layer of epithelium | 3/18 | 16% |
E-cadherin distribution in intercellular junctions denoted by arrows Ep – Epithelium, Ct – Connective tissue (40X).
Loss of expression of E- cadherin in inter cellular junctions in Cyclosporine A induced gingival overgrowth (arrows denote focal loss of E-cadherin (40x).
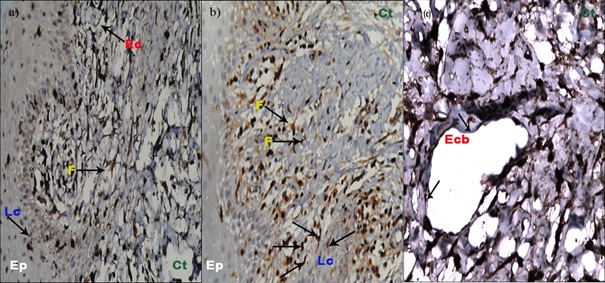
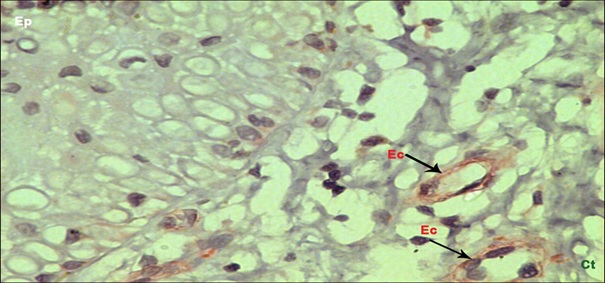
S100 A4 expression in healthy and DIGO samples: [Table/Fig-5] shows the pattern of distribution of S100 A4 in the samples analysed. All the 17 healthy samples (100%) revealed moderate staining of S100 A4 in the langerhans cells of the epithelium and fibroblasts and endothelial cells in the connective tissue [Table/Fig-6a]. In all the 18 DIGO samples (100%), a moderate to intense expression of S100 A4 was noted due to increased numbers of fibroblasts and increased number of blood vessels [Table/Fig-6b] Focal areas showed break in the continuity of endothelial lining in three out of 18 samples analysed (16%) [Table/Fig-6c].
Staining pattern and distribution of S100 A4 in group -I and Group -II.
| Group | S100A4 Staining intensity and distribution | No. of samples reported positive | % of samples reported positive |
|---|
| Healthy individuals (I) | Moderate staining in Langerhans cells of epithelium and fibroblasts and endothelial cells of connective tissue. | 17/17 | 100% |
| Drug Induced Gingival Overgrowth (DIGO)(II) | Moderate to intense staining in Langerhans cells of epithelium and fibroblasts and blood vessels in connective tissue. | 18/18 | 100% |
| Focal areas with break in continuity of endothelial lining | 3/18 | 16% |
(a) Section of healthy human gingiva showing S100 A4 expression in langerhans cells of the epithelium, fibroblasts and endothelial cells of the connective tissue (arrows denote positive staining in Langerhans cells, fibroblasts and endothelial cells) (20x); (b and c) section of Cyclosporine A induced gingival overgrowth showing intense expression of S100 A4 in endothelial lined blood capillaries and fibroblasts in the connective tissue (20x) and break in blood vessel wall (40x). Arrows denote intense positive staining in fibroblasts & endothelial cells along with a break in the endothelial lining. F – fibroblast, Lc – Langerhan cells, Ep – Epithelium, Ct – Connective tissue, Ecb – Endothelial capillary break, Ec – Endothelial cell.
α - SMA staining in healthy and DIGO samples: [Table/Fig-7] shows the pattern of distribution of α - SMA in the samples analysed. Moderate staining was observed only in endothelial lined blood vessels in the CT of all the 17 healthy samples analysed (100%) [Table/Fig-8]. In DIGO, intense staining was seen in the CT in endothelial lined blood vessels. Positive staining was seen in the fibroblasts in the CT [Table/Fig-9]. The quantification of alpha SMA positive fibroblasts in the DIGO samples was accomplished by cell counting of fibroblasts per field. The results revealed a mean number of 4.4±0.956 α – SMA positive cells per field in the DIGO samples [Table/Fig-7].
Staining pattern and distribution of SMA in Group -I & Group -II.
| Group | α SMA staining intensity and distribution | No. of samples reported positive | % of samples reported positive | Mean number of αSMA fibroblasts |
|---|
| Healthy individuals (I) | Moderate staining only in endothelial lined blood vessels of gingival connective tissue | 17/17 | 100% | 0 |
| DIGO (II) | Intense staining in endothelial lined blood vessels and fibroblasts of gingival connective tissue | 18/18 | 100% | 4.4±0.956 |
Section of healthy human gingiva showing expression of α-SMA in endothelial lined blood capillaries (arrow denotes positive staining in endothelial lined capillary wall) (40x). Ep – epithelium, Ct – Connective tissue, Ec – Endothelial cell.
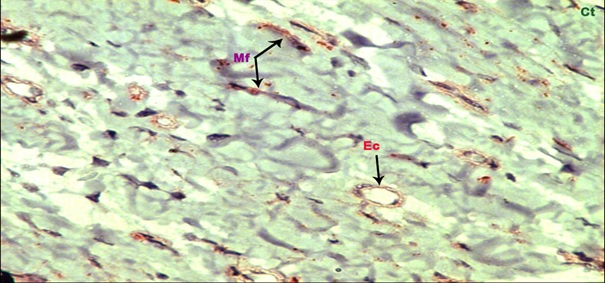
Section of Cyclosporine A induced gingival overgrowth showing intense staining of α-SMA in myofibroblasts (arrow denotes positive staining of myofibroblasts) (40x). Mf – Myofibroblast, Ec – Endothelial cell, Ct- Connective Tissue.
Discussion
The pathogenesis of DIGO is still inconclusive despite several advances in molecular research. The present study was carried out in an effort to determine if EMT operates in DIGO to serve as a source of fibroblasts. To confirm this mechanism, we sought to investigate the immunohistochemical expression of three specific markers for EMT namely E-Cadherin, S100 A4 and α-SMA which are known to play an important role in the mechanism of EMT. An earlier in vitro study by Sume [18], demonstrated that when primary human gingival epithelial cells were treated with TGF-β1 it induced a reduction barrier function as evidenced by reduced electrical resistance and paracellular permeability assays and simultaneously decreasing the expression of cell surface E-Cadherin. With this objective we went on to assess the expression of E-Cadherin, S100 A4 (FSP-1), and α – SMA in gingival tissue samples obtained from healthy individuals and patients with Cyclosporine induced gingival overgrowth. Moreover, α – SMA is a putative myofibroblast marker. Since myofibroblasts are implicated in EMT induced fibrosis we sought to analyse α – SMA expression in the samples. E-Cadherin is required for the maintenance of normal intercellular adhesion and barrier integrity in oral tissues and the junctional epithelium [19]. Jeopardized E-Cadherin expression due to transcriptional repression could alter the cell phenotype from epithelial to fibroblast with spindle shape morphology [20]. Okada H and co-workers have shown that, the epithelial cells migrate from the epithelial layer, travel through the basement membrane and accumulate in the interstitium of the tissue. Here they eventually get rid of all of their epithelial markers to gain a fully fibroblastic phenotype [21].
In our study of healthy samples, an intense staining of E-Cadherin was observed at the intercellular junctions in all layers of the epithelium along with an intact basement membrane. In contrast, reduced or absence of E-cadherin staining was observed in the basal and suprabasal layers of the epithelium of DIGO samples. These findings demonstrated in the present study could be the possible cause for the initial step of epithelial cells migrating into the CT. E-Cadherin is dependent on calcium for its function and cyclosporine being a calcium channel blocker could possibly affect the function of E-cadherin leading to loss of intercellular junctions and basement membrane. However, this finding needs further confirmation. S100 A4 was used in our study due to its positive association with cells undergoing EMT. S100 is a family of low molecular weight proteins. Members of S-100 family have been implicated in calcium signal transduction, cytoskeletal-membrane interaction, cellular growth & differentiation [22]. S100 A4 also called fibroblast specific protein has been implicated in maturation of Langerhans cells in the gingiva as shown by Anjana R et al., [23]. Expression of this marker has been demonstrated when epithelial cells were treated with cytokines which confirmed this as a unique marker of fibroblast lineage that gets expressed in the process of EMT [24]. FSP-1 has been found to be expressed occasionally in healthy renal tissues and increased expression has been observed in renal fibrosis by Iwano M and colleagues [25]. In our study S100 A4 was seen to be expressed in Langerhans cells, fibroblasts, endothelial cells and endothelial lined blood capillaries in healthy gingival tissue samples. However, in DIGO samples, an intense expression of S100 A4 in the CT was observed (predominantly in fibroblasts and endothelial cells) as compared to control. The increased staining of S100 A4 in fibroblasts in the CT of DIGO samples could be explained based on the fact that during active EMT, there is a distinct up regulation and acquisition of S100 A4 by fibroblasts which eventually contribute to fibrosis. Our findings are in accordance with a study by Yu CC et al., who demonstrated increased expression of S100 A4 in buccal mucosal biopsies from oral sub mucous fibrosis patients compared to healthy individuals [26]. Moreover, they also demonstrated up regulation of S100 A4 mRNA in areocoline treated human buccal fibroblasts proving the role of S100 A4 in the fibrosis process.
The positive expression of S100 A4 on endothelial cells and endothelial lined blood capillaries was an interesting and intriguing finding in our study since there is yet another pathological process called endothelial mesenchymal transition (End MT) where endothelial cells acquire the markers of mesenchymal cells and migrate into the adjacent stroma to become fibroblasts. Moreover in the present study we observed increased presence of blood vessels possibly because S100 A4 is an angiogenic factor as documented by Wang L and colleagues [27]. By virtue of the same proangiogenic nature, there exists a possibility of influx of circulating fibrocytes that could contribute to the mechanism of fibrosis. Also in 16% of the DIGO samples, a break in the blood vessel integrity was seen. These findings could lead us to hypothesize that endothelial cells disintegrate from the blood vessels and migrate to the CT transforming into fibroblasts. Hereditary Gingival Fibromatosis (HGF) is similar to DIGO except for the clinical picture and hereditary nature. A study by Bitu CC et al., demonstrated the presence of myofibroblasts in the above condition [28]. A specific marker for myofibroblasts is α-SMA. It is one of the six actin family members. In the adult, prominent α-SMA expression can be found in vascular smooth muscle cells and myoepithelial cells [29]. Type 2 EMT, which contributes to tissue fibrosis, is also associated with myofibroblasts that eventually express α-SMA. Increased α-SMA positive cells have been demonstrated in fibrosis of the kidney [30]. In our study, α-SMA staining was seen in the endothelial cells of healthy gingival tissue samples. In the DIGO samples, we observed an intense expression of α-SMA in perivascular smooth muscle cells and endothelial lining blood capillaries with a positive staining of few fibroblasts in and around the areas of fibrosis.
The mean number of α-SMA positive fibroblasts in the DIGO samples was found to be 4.4±0.956 per field. Our study findings are concurrent with those of Chung Y et al., who demonstrated that Cyclosporine treatment of gingival fibroblasts upregulated α-SMA, Collagen type 1, TGF beta and the Sonic Hedgehog at the genetic and protein level [31]. Myofibroblasts could be seen as a distinct subpopulation of fibroblasts that express the putative marker α-SMA. Their presence in DIGO may be suggestive of their role in fibrosis. A previous study examining the ultra structural properties of cyclosporine induced gingival overgrowth specimens found microscopic changes suggestive of the presence of myofibroblasts in this condition [32]. Shi Wen X and co workers have shown that TGF beta, a potent modulator of trans-differentiation of fibroblasts into myofibroblasts can up regulate the production of Endothelin-1 (ET-1) by lung fibroblasts which in turn can cause increased collagen production [33]. In addition, Hidekata Yasuoka et al., in their study has suggested that Insulin Growth Factor Binding Protein-5 (IGFBP-5) is overexpressed in idiopathic pulmonary fibrosis [34]. Interestingly a study by Ogunwobi and colleagues suggested that cyclooxygenase could be a viable target for treating EMT associated development and progression of hepatocellular carcinoma [35]. A previous study in our laboratory has shown that ET-1 mRNA and protein levels to be significantly higher in cyclosporine induced gingival overgrowth [36]. Increased IGFBP-5 expression [37] and elevated cyclooxygenase levels in Cyclosporine induced DIGO in our laboratory [38] have also been demonstrated proving the fact that these factors could contribute to EMT.
Limitation
The limitations of our study were that the sample size was limited and confocal microscopy could not be used due to non-availability that could have co-localized all the three antigens. Also, S100 A4 stained melanocytes along with Langerhans cells and there was inability to differentiate between the two. EMT is a molecular mechanism that has been found to operate in other fibrotic conditions. There are other markers that can be employed to understand the EMT mechanism. A study by Lin YH et al., has shown the up regulation of the EMT marker snail in gingival fibroblasts following cyclosporine administration both at the genetic level and protein level [39]. Many such studies employing various EMT markers are required. It may be worthwhile to develop pharmacological targets to these protein markers that cause EMT to prevent fibrosis of the gingiva and other vital organs.
Conclusion
Our study sheds light on the possible role played by epithelial mesenchymal transition in the pathogenesis of DIGO. In-vitro culture studies and animal models are further needed to elucidate the same. Therapeutic strategies based on preventing EMT could be applied in the future for the management of DIGO.
[1]. Hassell TM, Page RC, Lindhe J, Histologic evidence for impaired growth control in diphenylhydantoin gingival overgrowth in manArch Oral Biol 1978 23:381-84. [Google Scholar]
[2]. Ayanoglou CM, Lesty C, Cyclosporin A induced gingival overgrowth in the rat: a histological, ultrastructural and histomorphometric evaluationJ Periodontal Res 1999 34:7-15. [Google Scholar]
[3]. Wondimu B, Reinholt FP, Modeer T, Stereologic study of Cyclosporine A induced gingival overgrowth in renal transplant patientsEur J Oral Sci 1995 103:199-206. [Google Scholar]
[4]. Ballard JB, Bulter WT, Biochemical studies on the collagenous and non collagenous proteins of human gingivaJ Oral Pathol 1974 3:176-84. [Google Scholar]
[5]. Saito K, Mori S, Tanda N, Sakamoto S, Immunolocalizaiton of cMyc and bcl-2 proto-oncogene products in gingival hyperplasia induced by nifedipine and phenytoinJ Periodontol 2000 71:44-49. [Google Scholar]
[6]. Saito K, Mori S, Tanda N, Sakamoto S, Expression of p53 protein and Ki 67 antigen in gingival hyperplasia induced by Nifedipine and PhenytoinJ Periodontol 1999 70:581-86. [Google Scholar]
[7]. Quan TE, Cowper SE, Bucala R, The role of circulating fibrocytes in fibrosisCurr Rheumatol Rep 2006 8:145-50. [Google Scholar]
[8]. Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Haematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytesExp Haematol 2006 34:219-29. [Google Scholar]
[9]. Radisky DC, Epithelial-mesenchymal transitionJ Cell Sci 2005 118:4325-26. [Google Scholar]
[10]. Boyer B, Valles AM, Edme N, Induction and regulation of epithelial mesenchymal transitionsBiochem Pharmacol 2000 60:1091-99. [Google Scholar]
[11]. Lee JM, Dedhar S, Kalluri R, Thompson EW, The epithelial-mesenchymal transition: new insights in signaling, development, and diseaseJ Cell Biol 2000 172:973-81. [Google Scholar]
[12]. Huber MA, Kraut N, Beug H, Molecular requirements for epithelial mesenchymal transition during tumor progressionCurr Opin Cell Biol 2005 17:548-58. [Google Scholar]
[13]. Xue C, Plieth D, Venkov C, Xu C, Neilson E.G, The gatekeeper effect of Epithelial mesenchymal transition regulates the frequency of breast cancer metastasisCancer Res 2003 63:3386-94. [Google Scholar]
[14]. Zeisberg E.M, Potenta S, Xie L, Zeisberg M, Kalluri R, Discovery of endothelial to mesnchymal transition as a source for carcinoma associated fibroblastsCancer Res 2007 67:10123-28. [Google Scholar]
[15]. Salman BN, Vahabi S, Movaghar SE, Mahjour F, Proliferative and inductive effects of Cyclosporine a on gingival fibroblast of child and adultDental Research Journal 2013 10:52-58. [Google Scholar]
[16]. Angelopoulous AP, Goaz PW, Incidence of diphenylhydantoin gingival hyperplasiaOral Surg Oral Med Oral Pathol 1972 34:898-06. [Google Scholar]
[17]. Carrillo C, Penarrocha M, Penarrocha M, Vera F, Penarrocha D, Immunihisto-chemical study of langerhan cells in periapical lesions- correlation with inflammatory cells infiltration and epithelial cell proliferationMed Oral Patol Oral Cir Bucal 2010 15(2):e335-39. [Google Scholar]
[18]. Sume SS, Kantarci A, Lee A, Hasturk H, Trackman PC, Epithelial to mesenchymal transition in gingival overgrowthAm J Pathol 2010 177:208-18. [Google Scholar]
[19]. Fujita T, Hayashida K, Shiba H, Kishimoto A, Matsuda S, The expressions of claudin-1 and E-cadherin in junctional epitheliumJ Periodontal Res 2010 45:579-82. [Google Scholar]
[20]. Reichert M, Muller T, Hunziker W, The PDZ domains of zonula occludens-1induce an epithelial to mesenchymal transition of Madin- Darby canine kidney I cells. Evidence for a role of β-catenin/Tcf/Lef signalingJ Biol Chem 2000 275:9492-500. [Google Scholar]
[21]. Okada H, Strutz F, Danoff TM, Kalluri R, Neilson EG, Possible mechanisms of renal fibrosisContrib Nephrol 1996 118:147-54. [Google Scholar]
[22]. Barraclough R, Calcium-binding protein S100A4 in health and diseaseBiochem Biophys Acta 1998 1448:190-99. [Google Scholar]
[23]. Anjana R, Joseph L, Suresh R, Immunohistochemical localization of CD1a and S100 in gingival tissues of healthy and chronic periodontitis subjectsOral Dis 2012 18:778-85. [Google Scholar]
[24]. Okada H, Danoff TM, Kalluri R, Neilson EG, The early role of FSP1 in epithelial-mesenchymal transformationAm J Physiol 1997 273(4 Pt 2):F563-74. [Google Scholar]
[25]. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG, Evidence that fibroblasts derive from epithelium during tissue fibrosisJ Clin Invest 2002 110:341-50. [Google Scholar]
[26]. Yu CC, Tsai CH, Hsu HI, Chang YC, Elevation of S100 A4 expression in buccal mucosa fibroblasts by Arecoline: implication in the pathogenesis of oral submucous fibrosisPLoS one 2013 8:55122 [Google Scholar]
[27]. Wang L, Wang X, Liang Y, Diao X, Chen Q, S100 A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13Acta Biochem Pol 2012 59:593-98. [Google Scholar]
[28]. Bitu CC, Sobral LM, Kellermann MG, Martelli-Junior H, Zecchin KG, Graner E, Heterogeneous presence of myofibroblasts in hereditary gingivalfibromatosisJ Clin Periodontol 2006 33:393-400. [Google Scholar]
[29]. Beha G, Saril G, Brunetti B, Sassi F, Ferrara D, Benazzi C, Morphology of the myoepithelial cell: immunohistochemical characterization from resting to motile phaseScientific World Journal 2012 2012:252034 [Google Scholar]
[30]. Slattery C, Campbell E, McMorrow T, Ryan MP, Cyclosporine A Induced Renal Fibrosis -A Role for Epithelial-Mesenchymal TransitionAm J Pathol 2005 167:395-407. [Google Scholar]
[31]. Chung Y, Fu E, Chin YT, Tu HP, Chiu HC, Shen EC, Role of Shh and TGF beta in cyclosporine enhanced expression of collagen and alpha SMA by gingival fibroblastsJ Clin Periodontol 2015 42:29-36. [Google Scholar]
[32]. Yamasaki A, Rose GG, Pinero GJ, Mahan CJ, Ultrastructure of fibroblasts in cyclosporin A-induced gingival hyperplasiaJ Oral Pathol 1998 16:129-34. [Google Scholar]
[33]. Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Constitutive ALK5-independent c-jun n-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin a and b receptorsMol Cell Biol 2006 26:5518-27. [Google Scholar]
[34]. Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Augustine MK Choi, Feghali-Bostwick CA, Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltrationAm J Pathol 2006 169:1633-42. [Google Scholar]
[35]. Ogunwobi OO, Wang T, Zhang L, Liu C, Cyclooxygenase-2 and Akt mediate multiple growth-factor-induced epithelial-mesenchymaltransition in human hepatocellular carcinomaJ Gastroenterol Hepatol 2012 27:566-78. [Google Scholar]
[36]. Tamilselvan S, NarayanaRaju S, Loganathan D, Kamatchiammal S, Abraham G, Suresh R, Endothelin-1 and Its Receptors ETA and ETB in Drug-Induced Gingival OvergrowthJ Periodontol 2007 78:290-95. [Google Scholar]
[37]. Subramani T, Sakkarai A, Senthilkumar K, Periasamy S, Abraham G, Rao S, Expression of insulin like growth factor binding protein-5 in drug induced human gingival overgrowthIndian J Med Res 2007 125:43-48. [Google Scholar]
[38]. Rao SR, Balaji TM, Prakash PS, Lavu V, Elevated levels of cyclooxygenase 1 and 2 in human cyclosporine induced gingival overgrowthProstaglandins other Lipid Mediat 2014 113-115:69-74. [Google Scholar]
[39]. Lin YH, Yu CC, Lee SS, Chang YC, Elevated snail expression in human gingival fibroblasts by Cyclosporine A as the possible pathogenesis for gingival overgrowthJ Formos Med Assoc 2015 114:1181-86. [Google Scholar]