The developement of dental caries is a dynamic process involving cycles of demineralization and remineralization. Demineralization results in the loss of calcium and phosphate ions creating a subsurface lesion. Remineralization utilizes the existing calcium and phosphate ions in saliva aided by available salivary fluoride to create a new surface on existing crystal remnants in the subsurface lesion. Sub parts per million (ppm) of salivary fluoride levels prevent dental caries by shifting the balance from demineralization to remineralization at the tooth-oral fluid interface, due to the precipitation of calcium phosphates and the formation of fluorohydroxyapatite in tooth structure. The ability of fluoride to affect the demineralization-remineralization process depends on whether fluoride is available in the oral cavity at the right time and proper concentration. Maintaining low levels of fluoride release over long periods is important in the inhibition of demineralization and the promotion of remineralization [1].
The rationale for caries preventive effect of fluoride has been known for many decades. The fact that fluoride can be incorporated into the crystalline lattice of dental hard tissues, resulting in a tissue less soluble in acid environment, has been the scientific corner stone for caries prevention [2].
For many years professionally applied topical fluorides have been used effectively to prevent caries, especially in children. Professionally available fluoride is in the form of gels, foams, (containing around 12,300 ppm of fluoride) used effectively for inhibiting dental caries especially in children. The fluoride varnishes have gained immense popularity in the field of pediatric dentistry due to its ease of application thereby facilitating its use in precooperative children, patients with exaggerated gag reflex, those demanding special health care needs and also in children exposed to head and neck radiation [3].
Recent studies have shown the use of fluoride varnishes to be effective in the prevention of early childhood caries and reduce caries by 25%-45% [4,5].
According to American Dental Association council on scientific affairs concluded that fluoride varnish should be applied every six months as it is effective in reducing caries prevalence in high risk populations and also prevents caries in primary as well as permanent dentitions of children and adolescents [6]. Fluoride levels in saliva after application of fluoride varnish are influenced by different parameters like initial fluoride concentration applied, time since exposure, fluoride retention, delivery method and fluoride clearance from the oral cavity [1]. The greatest release of fluoride occurs in the first three weeks and then tapers [7–9].
Newly marketed fluoride varnishes are supposed to release fluoride slowly and for extended periods of time. The present study intends to determine the fluoride release from three different fluoride varnishes (representing new generation and conventional varnishes) over a period of time through salivary fluoride estimation.
Materials and Methods
This in-vitro prospective study was conducted in the Department of Pedodontics and Preventive Dentistry, College of Dental Sciences, Davangere, Karnataka, India, to determine the longevity of fluoride release from three different fluoride varnishes for a period of 6 months using fluoride ion electrode. Twenty four extracted primary anterior teeth with sound tooth structure were included.
The teeth were stored in saline till they were used for this study, and then the teeth were cleaned and dried with gauze. The tooth surfaces were then covered by nail varnish except for a 3mm X 3mm window on the facial (labial) surface of crown, where the test material was applied.
The test material used were ClinproTM XT Varnish [3M ESPE], Fluoritop SR [ICPA] and Fluorprotector varnish [Ivoclar Vivadent].
The teeth were randomly divided into four groups of six each. Three groups correspond to test products and 4th group comprised of untreated controls. In each group, the test material was applied following manufacturer’s instructions. Fluorprotector and Fluoritop SR varnishes were applied with the brush provided inside the package. ClinproTM XT Varnish was mixed as per manufacturer’s instructions, applied on the tooth and then light cured for 20 seconds. The teeth were then placed in individual plastic containers at room temperature, containing artificial saliva at a pH of 7.2. The teeth were removed from plastic container and placed in new plastic container containing fresh artificial saliva sequentially at 1 day, 1 month, 3 months and 6 months after the application of fluoride varnish. After the transfer, the solution from preceding plastic container was taken for fluoride analysis. Fluoride ion concentration was measured by ion selective electrode for fluoride, calibrated with Total Ionic Strength Adjustment Buffer (TISAB III) and fluoride standards. This method measured fluoride ion concentration in parts per million (ppm) in solution.
Fluoride release at each time interval for different groups was assessed by repeated measures ANOVA. One-way ANOVA was used for multiple group comparisons followed by Post hoc Tukey’s Test for group wise comparisons.
Results
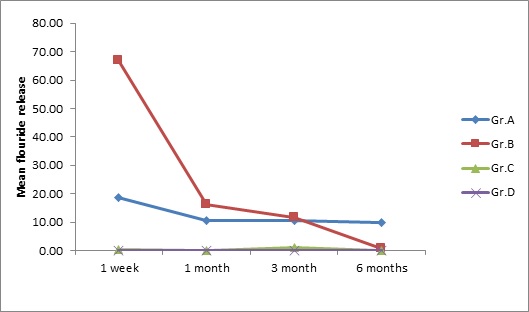
The results of the present study showed that the ClinproTM XT Varnish (9.78±4.11) had consistently and substantially more fluoride release than Fluoritop SR (0.61±0.36) and Fluorprotector (0.17±0.02) over an extended period of time [Table/Fig-1a,1b]. Fluorprotector showed the lowest rate of fluoride release among all the groups compared. Although all fluoride varnishes released fluoride, the greatest release was observed during 1st week. The results of the study were statistically significant (p<0.05) [Table/Fig-2,3].
Fluoride release from four groups.
| Group | 1 Week | 1 Month | 3 Months | 6 Months | ANOVA |
|---|
| F | p-value |
| ClinproTM XT Varnish | 18.78±7.35 | 10.59±4.19 | 10.55±5.01 | 9.78±4.11 | 3.823 | 0.03 * |
| Fluoritop SR | 66.92±16.30 | 16.31±13.67 | 11.75±10.07 | 0.61±0.36 | 37.52 | 0.01 * |
| Fluorprotector | 0.27±0.30 | 0.06±0.03 | 1.05±1.10 | 0.17±0.02 | 3.716 | 0.03 * |
| Control | 0.015±0.004 | 0.013±0.003 | 0.020±0.011 | 0.015±0.004 | 1.378 | 0.28 |
Repeated measures ANOVA
*p<0.05, Significant
Rate of fluoride release from different fluoride varnishes at different time interval.
Gr. A – ClinproTM XT vanish, Gr. B - Fluoritop SR
Gr. C - Fluorprotector, Gr. D – Control
Intergroup comparison of the rate of fluoride release.
| Group | 1 Week | 1 Month | 3 Months | 6 Months |
|---|
| Mean±SD | Mean±SD | Mean±SD | Mean±SD |
|---|
| 1. ClinproTM XT Varnish (C.XT) | 18.78±7.35 | 10.59±4.19 | 10.55±5.01 | 9.78±4.11 |
| 2. Fluoritop SR(F.SR) | 66.92±16.30 | 16.31±13.67 | 11.75±10.07 | 0.61±0.36 |
| 3. Fluorprotector(FP) | 0.27±0.30 | 0.06±0.03 | 1.05±1.10 | 0.17±0.02 |
| 4. Control© | 0.015±0.004 | 0.013±0.003 | 0.020±0.011 | 0.015±0.004 |
| ANOVA | F | 74.60 | 7.68 | 7.14 | 32.09 |
| p | 0.01 * | 0.001 * | 0.002 * | 0.01* |
* p < 0.05, Significant
Post hoc tukey’s test done for different groups of varnishes at different time interval. (* p < 0.05, Significant)
| Groups Compared | Mean diff | p-value | Mean diff | p-value | Mean diff | p-value | Mean diff | p-value |
|---|
| ClinproTM XT vs. Fluoritop-SR | 48.14 | 0.01 * C.XT< F.SR | 5.72 | 0.52 | 1.2 | 0.98 | 9.17 | 0.01* C.XT > F.SR |
| ClinproTM XT vs. Fluorprotector | 18.54 | 0.03 * C.XT > FP | 10.53 | 0.09 | 9.5 | 0.04,* C.XT> FP | 9.62 | 0.01* C.XT > FP |
| ClinproTM XT vs. Control | 18.77 | 0.008 * C.XT >© | 10.57 | 0.08 | 10.53 | 0.02,* C.XT >© | 9.77 | 0.01* C.XT >© |
| Fluoritop SR vs. Fluorprotector | 49.8 | 0.01* F.SR >FP | 16.25 | 0.004 * F.SR>FP | 10.71 | 0.02,* F.SR >FP | 0.44 | 0.98 |
| Fluoritop SR vs. Control | 66.9 | 0.01* F.SR >© | 16.3 | 0.004 * F.SR>© | 11.73 | 0.009 * F.SR >© | 0.59 | 0.95 |
| Fluorprotector vs. Control | 0.26 | 1.00 | 0.05 | 1.00 | 1.03 | 0.99 | 0.15 | 0.99 |
Discussion
Dental caries rates may have declined rapidly in the last 30 years especially in the developed nations, but in India and many other developing countries, dental caries still remain the most prevalent oral disease. For many children, the prevention of dental caries is still an important and time consuming task [10].
The use of topically applied fluoride in various vehicles has produced major reductions in prevalence and incidence of dental caries and has shown to be both safe and effective [1]. The benefits of fluoride in preventing dental caries have been known for over 65 years and it is one of the most researched topics in dentistry.
Fluoride plays a major role in caries prevention by inhibiting demineralization, enhancing remineralization, and inhibiting plaque bacteria. Common fluoride delivery system includes water, toothpaste, supplements, mouth rinses, gels, foams, mouse and varnish. All have variable fluoride concentration, ranging from 0.5 to 22,600 sub ppm [11].
Fluoride varnishes have replaced topical gel treatments in many countries. For more than 25 years, fluoride varnishes have been the standard of practice for the professional application of topical fluoride [4]. Fluoride varnish covers the teeth as an adherent film that lasts for 24 hours. Fluoride varnishes were developed to prolong the contact time between fluoride and enamel.
The first fluoride varnish was marketed in Europe in 1964. The US Food and drug administration approved the use of fluoride varnish for dentistry in 1994 to be used as cavity liner or desensitizing agent [1]. Application of fluoride varnish results in formation of calcium fluoride globules on the tooth surface, which are stabilized by intraoral protein phosphates and act as an insoluble reservoir at neutral pH. During a cariogenic challenge the pH is reduced, resulting in dissolution of calcium fluoride globules. The calcium-phosphate ion solubility is lower releasing fluoride and increasing the saturation of calcium phosphate in saliva and plaque. This aids in preventing the dissolution of calcium phosphate from tooth mineral and increases the rate of remineralization [1].
Numerous studies have shown fluoride varnishes to be clinically effective [12–17]. A recent review of fluoride varnishes by Beltran-Anguilar and colleagues summarizes their clinical use, cariostatic mechanism, efficacy, safety and toxicity [18].
Recent studies have shown that the use of fluoride varnishes to be effective in the prevention of early childhood caries and reduce caries by 25%-45% [4,5]. They have been shown to decrease incidence of root caries and to be better than other topical fluoride agents [3].
Also the effectiveness and safety of fluoride varnish has been validated in more than 50 clinical trials, including several meta-analyses and systematic reviews [19–23]. Manufacturers vary in their recommendations for resuming routine oral hygiene after the application of fluoride varnishes. They range from waiting 4-6 hours to the next morning. Clinical recommendations by authors on resuming tooth brushing after fluoride varnish application varies from 12 hours to 24 hours [4]. Some of the major advantages of fluoride varnishes include ease of application, rapid setting, prolonged contact time, slow fluoride release, smaller amount required for entire dentition, rare complications and minimal risk of fluorosis [1].
Some of the commonly marketed conventional fluoride varnishes are Duraflor, Duraphat, Fluoritop SR, Enamel pro varnish, Colgate Prevident, Omni varnish etc., and the newer fluoride varnish is ClinproTM XT Varnish which is a light cured resin modified glass ionomer that releases calcium, fluoride and phosphorus.
All the fluoride varnishes release fluoride but the rate of fluoride release is not constant, it varies. This is probably because of inherent differences in the carriers for the fluoride in commercially available fluoride varnishes, which affects the rate of release of fluoride. This variation in fluoride release by different varnishes has been previously reported [9]. The carrier in the varnish is the component which is held proprietary by manufacturers, and properties of this component of fluoride varnish are what appears to make the difference in the fluoride release. Various authors have studied the difference in the rate of fluoride release in different topical fluorides [Table/Fig-4].
Studies of fluoride release from different topical fluoride agents.
| Group | 1 Week | 1 Month | 3 Months |
|---|
| Castillo JL [25] | To evaluate fluoride release from two Fluoride varnishes. | DuraphatDuraflor | Duraphat released more fluoride than Duraflor and also there was great variability in release of fluoride in both samples. However both released fluoride for 5-6 months. |
| Castillo JL [7] | To evaluate fluoride release from varnishes in two different protocols. | Duraphat | Total release of fluoride was significantly higher in three application regimen than in single application. |
| Eakle WS [8] | To examine the concentration of fluoride in whole saliva following application of fluoride varnish or single rinse with fluoride solution. | 0.05% Sodium fluoride solution and 5.0% of sodium fluoride varnish. | Maximum fluoride levels were significantly greater with varnish than with the rinse and remained above baseline levels for a longer duration. |
| Ritwik P [3] | To compare the rate of fluoride release from fluoride varnishes over a 48-hour period and ascertain the time at which a plateau occurred. | Premier Enamel Pro varnish (EP), Colgate prevident (CP), Omni Vanish (OV), and Omni Vanish XT (OVXT). | CP, EP, and OV released maximum rate of fluoride in the first 4 hours whereas OVXT did not have plateau. The studied varnishes released different concentrations of fluoride despite the fact that they all contained 5% sodium fluoride. |
| Jablonowski BL [1] | To compare the amount and rate of fluoride release of new fluoride varnishes with other traditional fluoride varnishes. | Enamel Pro, Duraphat, Vanish, and Vanish XT | Enamel Pro had a greatest cumulative fluoride release. There was no significant difference between Duraphat and Vanish. Vanish XT had the lowest cumulative fluoride release. |
So the aim of our study was to determine the longevity of fluoride release from three different fluoride varnishes (ClinproTM XT Varnish; 3M ESPE, Fluoritop SR; ICPA Health Products Ltd., Fluorprotector, Ivoclar Vivadent.) through salivary fluoride estimation.
ClinproTM XT Varnish is a light cured resin modified glass ionomer that releases fluoride, calcium, and phosphates. The manufacturers states that ClinproTM XT Varnish releases more fluoride in 1st hour than conventional varnishes and releases fluoride for over 6 months [24]. We agree with the manufacturer as in our study ClinproTM XT Varnish had constant rate of fluoride release over a period of 6 months. In the 1st week of our study ClinproTM XT Varnish released 18.78 ± 7.35ppm of fluoride which gradually decreased to 9.78 ± 4.11ppm after 6 months [Table/Fig-1]. Although the fluoride release was reduced but still it maintained a constant release of fluoride till 6 months in contrast to Fluoritop SR which showed highest fluoride release for 1st week 66.92 ± 16.30ppm of F which rapidly decreased to 0.61 ± 0.36 at the end of 6 months [Table/Fig-1].
The results of this in-vitro study indicated that the newly marketed fluoride varnish (ClinproTM XT Varnish) had significantly different fluoride release profiles compared with two conventional fluoride varnishes (Fluoritop SR and Fluorprotector). The rate of fluoride released into artificial saliva differed significantly according to the type of varnish. This is probably due to differences in resin carriers or additives used by the manufacturers, which may have an effect on fluoride release.
All of the fluoride varnishes tested in our study released fluoride for an extended period of time, with the greatest release occurring in the 1st week.
Salivary fluoride levels with rinse returned to baseline, on average in 2 hours while they remained elevated for 24 hours with the varnish [8]. Similar results to our study was obtained by Beth L. Joblonowski [1] and also by Castillo and Milgrom [7] who in their experimental study found that the fluoride varnishes released fluoride for an extended period of time, with the greatest occurring in the first three weeks.
In other study where the rate of fluoride release was assessed between Duraflor, Duraphat and Cavity shield, they saw rapid release of fluoride in first 7 hours and slower release thereafter. In their study maximum fluoride release was by Duraflor when compared to Cavity shield and Duraphat [9].
Similar study was done by Castillo who evaluated rate of fluoride release from Duraflor and Duraphat [25]. He saw that initial three week period; rate of release of Duraflor was much higher than that of Duraphat. But the mean absolute level of fluoride release was consistently higher for Duraphat than it was for Duraflor during six month evaluation.
For all the varnishes tested in our study, the greatest amount of fluoride was released soon after application, which coincides with in vivo [8,26] and in vitro studies [7]. This suggests a correlation between the fluoride found in the varnish and fluoride levels detected in human and artificial saliva after application.
In our study ClinproTM XT varnish released consistently and substantially more fluoride than Fluoritop SR and Fluorprotector in the period of 6 months. This is probably due to the chemical bond between glass ionomer of ClinproTM XT varnish and tooth structure. Based on resin modified glass ionomer technology, fluoride release would be slower and extended [27]. Another study done by Ritwik et al., has a similar finding as our study, where he used Omni Vanish XT which is also a glass ionomer based varnish which exhibited a sustained release of fluoride, although the initial rate of release in 1st 4 hours was lower than other products tested [3].
Fluoritop SR contains 50mg sodium fluoride per ml equivalent to 22.6mg of fluoride in slow release form. In our study fluoride release was highest by Fluoritop SR in 1st week 66.92 ± 16.30ppm and reduced thereafter to 0.61 ± 0.36ppm in six months. Though there was sudden drop in fluoride release but still it released fluoride for six months.
Fluoprotector which contains 0.9 percent difluorosilane by weight (1,000ppm F) in polyurethane-based varnish showed lowest rate of fluoride release. It was 0.27 ± 0.30ppm in 1st week and 0.17 ± 0.02ppm at the end of six months [Table/Fig-1]. Similar results to our study was found by Munshi et al., where he saw that Fluorprotector showed least values of calcium and phosphorus dissolutions compared to Bifluorid 12 and Fluoritop-SR. He suggested that this may be attributed to low fluoride content of Fluorprotector [10]. A significant elevation of fluoride levels in whole saliva was seen with Bifluorid [12], 1 hour after application of fluoride varnish, but the elevation was insignificant with Fluorprotector according to Tweetman and colleagues [26].
The fluoride ion concentration in control specimen was 0.015 ± 0.004 ppm at 1st week and same at six months [Table/Fig-1] which did not show much variation with time.
This study compared novel fluoride varnish (ClinproTM XT Varnish) with traditional agents (Fluoritop SR and Fluorprotector), wherein ClinproTM XT Varnish released consistently and substantially more fluoride than other tested products. So the clinicians should be encouraged to use such novel fluoride varnishes that can contribute to caries prevention, so that it will allow for the continuous presence of low fluoride levels at the plaque-enamel interface.
Limitation
There are limitations to using the data from our study directly in a clinical practice. This in-vitro study measured only the rate of fluoride release. It did not study the fluoride uptake by enamel. By virtue of it being an in-vitro study using artificial saliva, the dynamics of human saliva affecting the rate of F release were not considered. However, the data from this in-vitro study provides the clinician the knowledge of fluoride varnishes which releases fluoride over extended time period so that the selection of varnish can be made based on each individual patient clinical presentation and provider preference.
Conclusion
On the basis of the results obtained from this study, it is concluded that ClinproTM XT Varnish released consistently and substantially more fluoride than Fluoritop SR and Fluorprotector over an extended period of time. The newly marketed novel fluoride varnish (ClinproTM XT Varnish) had significantly different fluoride release from the two conventional fluoride varnishes (Fluoritop SR and Fluorprotector). Fluorprotector had the lowest rate of F release among all the fluoride varnishes compared.
Thus, within the limitations of the present study it can be concluded that clinicians should be encouraged to use such novel fluoride varnishes that will allow for continuous presence of low fluoride levels that will contribute to caries prevention.
Repeated measures ANOVA
*p<0.05, Significant
* p < 0.05, Significant