Intracranial space occupying lesions (ICSOL) can be of varied aetiology like infectious, neoplastic and inflammatory. Establishing an accurate neoplastic aetiology is essential for timely diagnosis and neurosurgical intervention [1]. With the development of recent investigative techniques in India during the past two decades, it has become obvious that brain tumours are as common in this country as elsewhere in the world [2].
Most of the patients with neoplasms have a fairly characteristic presentation. However, many patients with intracranial masses present a greater diagnostic challenge because of atypical presentation secondary to intratumoural haemorrhage, arterial occlusion and cerebral infarction or tumour involvement of silent areas. In such cases it is important to utilize modern neuro -radiological procedures like CT scan and MRI in order to detect the lesion to localize it [2].
The central CNS tumours that predominate in adults differ from those seen in children. The annual incidence of CNS tumours ranges according to published Western data from 10 to 17/100,000 persons for intracranial tumours and from 1 to 2/100,000 persons for intraspinal tumours. Of these about half are primary tumours and the rest are metastatic. Tumours of the CNS account for 20% of all cancers of childhood. Malignant CNS tumours are the second commonest cause of death from cancer in the under 15 year age group in both males and females [3].
This gives rise to urgent need for accurate characterization and localization of space-occupying lesions by cytology and histology, many a times requiring special diagnostic techniques such as immunohistochemical staining for detection of cell of origin [4]. Haematoxylin-eosin staining is still the mainstay for histopathologic diagnosis even in CNS tumours. However, Glial Fibrillary Acidic Protein staining can firmly establish the astrocytic origin and differentiation of a CNS neoplasm [5]. The exact histological diagnosis of CNS tumours is essential to predict the prognostic factors. The new diagnostic criteria and techniques have affected the relative frequencies of CNS tumours [6].
The present study attempts to provide preliminary data on morphological patterns of intracranial lesions in North Karnataka region and to study clinicopathological spectrum with correlation of radiological findings of ICSOL. Special emphasis is made on the utility of special stains and IHC markers in CNS tumours.
Materials and Methods
This prospective and retrospective study was carried out in the Department of Histopathology, Mahadevappa Rampure Medical College, Kalaburagi. A total of 62 biopsy specimens of intracranial lesions received from Department of Neurosurgery, Basaveshwara Teaching and General Hospital were included, during the period between January 2012 to June 2013 retrospectively and July 2013 to June 2015 prospectively.
Inclusion Criteria
All ICSOL were included in the study.
Exclusion Criteria
Inadequate biopsy specimen, haematoma, traumatic lesions, bony lesions of skull, demyelinating diseases, degenerative lesions, pineal gland and spinal cord lesions were excluded.
Biopsy specimen of CNS lesions were preserved in 10% formalin and allowed to fix for 24 hours. The haematoxylin and eosin stained sections of the CNS lesions were obtained by routine processing and paraffin embedding. Special stains and Immunohistochemistry were done wherever appropriate. The technique for IHC included antigen retrieval in tris buffer in a retriever blocking endogenous peroxidase with 3% hydrogen peroxide, incubating with primary mouse monoclonal antibody, developing chromogen with Diaminobenzidine (DAB) and counterstaining with haematoxylin. The immunostained slides examined for a panel of markers.
Clinical history of all cases was collected in a pretested proforma meeting the objectives of the study. Informed consent was obtained from all the patients who underwent surgery. Tumours of CNS tabulated according to the classification and grading of World Health Organisation. Statistical analysis was performed using Microsoft Excel Software and the Standard Statistical Package for the Social Sciences (SPSS) version 20.0 for windows. This study was approved by institution ethical committee.
Results
In a total of 62 cases of ICSOL, 50 cases (80.6%) were neoplastic and 12 cases (19.4%) non-neoplastic [Table/Fig-1]. Mean age was 26.72± 11.2 (range: 0.4 to 80) years. Males represented 29 (46.8%) of the study population, while 33 (53.2%) were females, with male to female ratio of (1:1.14). The peak age was seen in 31 to 40 years which accounted for 17(27.4%) cases [Table/Fig-2]. No statistical significant difference was obtained between males and females for all age groups (χ2 =1.75, p> 0.05).
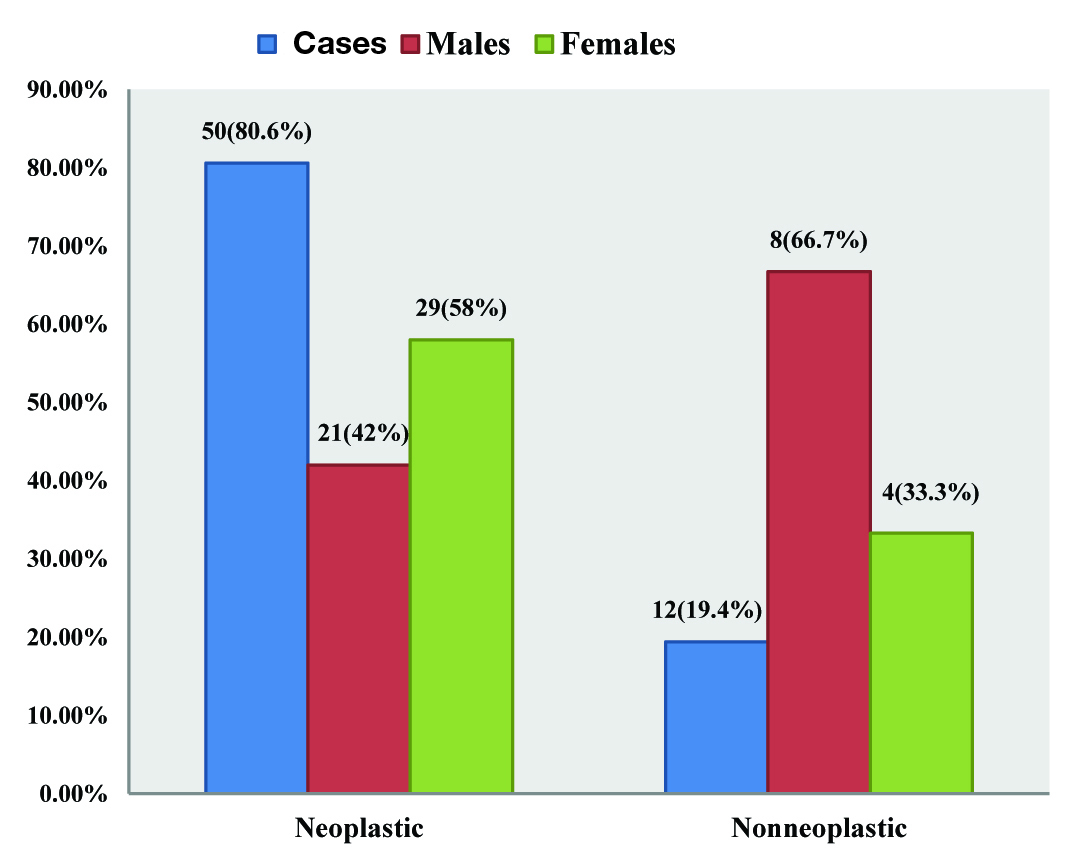
Age and Sex distribution of Histopathological types of ICSOL.
| ICSOL | Age (Years) |
|---|
| < 1 | 1-10 | 11-20 | 21-30 | 31-40 | 41-50 | 51-60 | >60 | Total | Percentage |
|---|
| Neoplastic lesions | M/F | M/F | M/F | M/F | M/F | M/F | M/F | M/F | M/F | |
|---|
| Astrocytoma | - | 2 /1 | 4/2 | 1/5 | 1/1 | 0/1 | 1/1 | 1/1 | 10/12 | 44% |
| Schwannoma | - | - | 0/1 | 1/0 | 1/3 | 1/0 | - | - | 3/4 | 14% |
| Meningioma | - | - | - | - | 0/1 | 0/3 | 0/1 | 0/1 | 0/6 | 12% |
| Embryonal tumours | - | 1/1 | - | - | - | - | - | - | 1/1 | 4% |
| Haemangioblastoma | - | - | - | - | 1/1 | - | - | - | 1/1 | 4% |
| Histiocytic tumours | - | 2/0 | - | - | - | - | - | - | 2/0 | 4% |
| Metastatic tumours | - | - | - | - | - | - | 1/0 | 1/0 | 2/0 | 4% |
| Mixed glial tumours | - | - | - | - | 1/1 | - | - | - | 1/1 | 4% |
| Pituitary adenoma | - | - | - | 0/1 | - | - | - | - | 0/1 | |
| Mature teratoma | - | - | - | 0/1 | - | - | - | - | 0/1 | |
| MPNST | - | - | - | - | 1/0 | - | - | - | 1/0 | |
| Choroid plexus papilloma | 0/1 | - | - | - | - | - | - | - | 0/1 | |
| Epidermoid tumour | - | - | - | - | 0/1 | - | - | - | 0/1 | |
| Subtotal | 0/1 | 5/2 | 4/3 | 2/7 | 5/8 | ¼ | 2/2 | 2/2 | 21/29 | |
| Nonneoplastic lesions |
| Cysts | 1/0 | - | 0/1 | - | ½ | - | 1/0 | - | 3/3 | 50% |
| Cerebral abscess | - | 2/1 | - | - | 1/0 | - | - | - | 3/1 | 33% |
| Infarct | - | - | - | 1/0 | - | - | - | - | 1/0 | |
| Reparative granuloma | - | - | - | 1/0 | - | - | - | - | 1/0 | |
| Subtotal | 1/0 | 2/1 | 0/1 | 2/0 | 2/2 | - | 1/0 | - | 8/4 | |
M=Male, F=Female
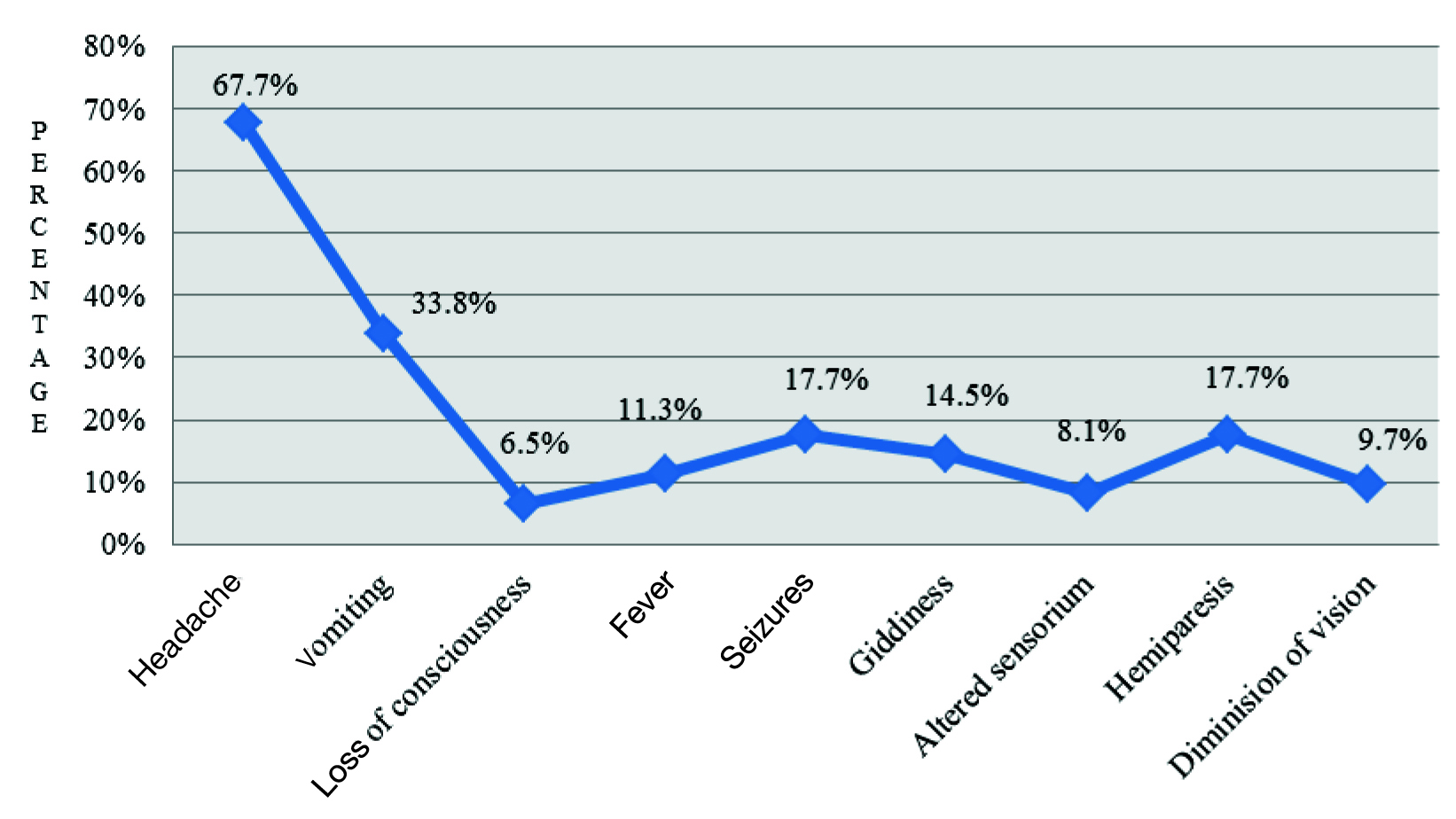
During clinical examination, majority of the patients presented with headache (67.7%) followed by vomiting, haemiparesis, seizures, giddiness, fever, altered sensorium, reduced vision and loss of conscious [Table/Fig-3]. Frontal lobe (16%) was the predominant site involved in most of the intracranial lesions, followed by cerebellopontine angle (14.5%) and temporal lobe (13%).
Presentation of Symptoms of ICSOL
A total of 50 CNS tumours were diagnosed during the study period. Of these, 48 (96%) cases were primary and the rest two (4%) cases were metastatic. Among the primary tumours, 24(50%) cases of gliomas constituted the largest category with eight (16.7%) cases of pilocytic astrocytoma and GBM each.
Astrocytoma comprised the highest percentage of the total tumours accounting for 22(44%) cases followed by schwannomas and meningothelial tumours which constituted the second and third group accounting for seven (14%) cases and six (12%) cases respectively. Female preponderance was noted in astrocytoma, schwannoma and meningioma [Table/Fig-2]. Highly statistical significant difference was present in the distribution of cases among different types of neoplastic lesions (χ2=22.43, p< 0.001).
According to WHO grading of gliomas, majority were grade I accounting for nine cases (37.5%) followed by eight cases of grade IV with 33.3% including a variant of GBM, then grade II with four cases (16.7%) and three cases (12.5%) of grade III. Among tumour of meninges, majority were grade I with seven cases (87.5%) followed by a single case of grade II [Table/Fig-4]. No statistical significant difference was present in the distribution of cases according to the grading of gliomas and tumour of meninges (χ2=1.07, p>0.05).
WHO grading of gliomas and tumour of meninges.
| WHOgrade | Gliomas | No of cases | Total | Percentage |
|---|
| I | Pilocytic Astrocytoma | 08 | 09 | 37.5% |
| I | SEGA | 01 |
| II | Diffuse Astrocytoma | 03 | 04 | 16.7% |
| II | Oligoastrocytoma | 01 |
| III | Anaplastic Astrocytoma | 02 | 03 | 12.5% |
| III | Anaplastic Ganglioglioma | 01 |
| IV | GBM | 07 | 08 | 33.3% |
| IV | Gliosarcoma | 01 |
| Sub Total | | 24 | |
| Tumour of Meninges |
| I | Meningothelial Meningioma | 03 | 07 | 87.5% |
| I | Secretory Meningioma | 01 |
| I | Fibroblastic Meningioma | 01 |
| I | Haemangioblastoma | 02 |
| II | Atypical Meningioma | 01 | 01 | |
| Subtotal | | 08 | |
On IHC, out of 33 cases of CNS tumours, 22 cases of astrocytic tumours were positive for GFAP along with a single case of Gliosarcoma, where vimentin showed positive in mesenchymal component and GFAP positive in neoplastic astrocytes with MIB-1 labelling >18% [Table/Fig-5]. An interesting case of Atypical teratoid /rhabdoid tumour showed positivity for GFAP, vimentin, S-100 and loss of INI nuclear expression noted [Table/Fig-6]. Both the cases of Cerebellar haemangioblastoma were CD34 positive [Table/Fig-7]. A single case of Malignant Peripheral Nerve Sheath Tumors (MPNST) showed positivity for S-100, vimentin whereas GFAP and Desmin negative [Table/Fig-8]. Histiocytic tumours showed positivity in the Langerhans cells for CD 1a [Table/Fig-9]. In case of Secretory meningioma, CEA showed positivity in the hyaline globules [Table/Fig-10]. Among the two cases of mixed glial tumours, GFAP was positive in both cases along with S-100 positivity seen in mature ganglion cells in case of Anaplastic Ganglioglioma [Table/Fig-11]. TTF 1 positivity in case of metastatic adenocarcinoma, documented that the primary was from the lung malignancy [Table/Fig-12]. S-100 showed positivity in Schwannoma.
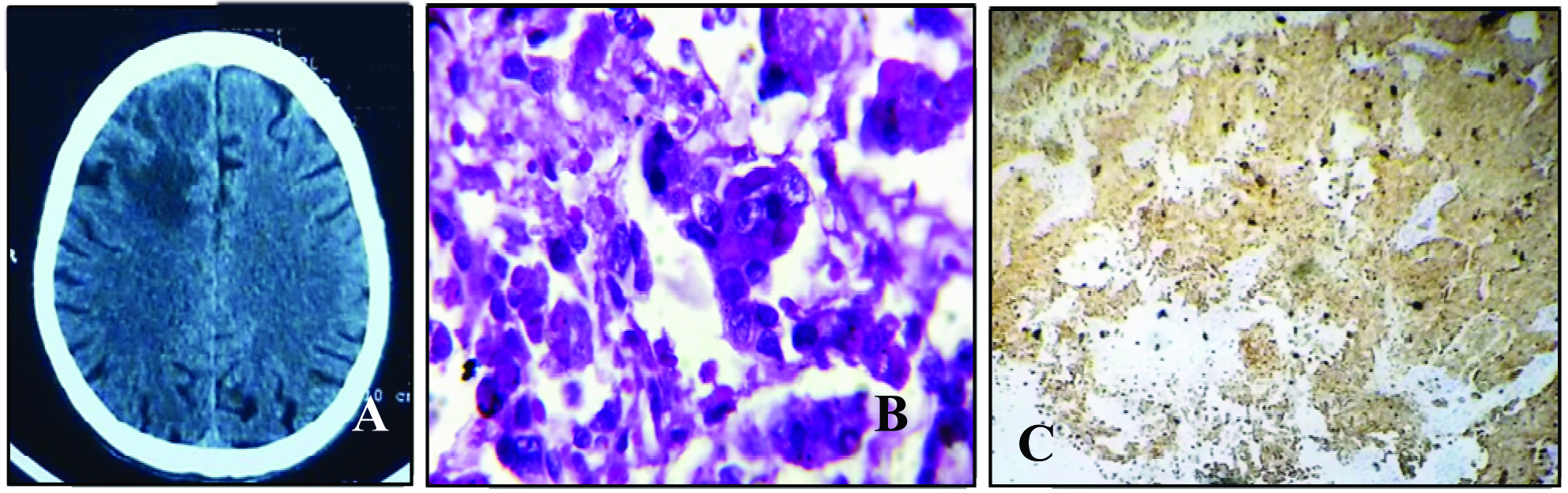
Gliosarcoma.
A= MRI showing a large mass with midline shift to right in the left parieto occipital region.
B= GFAP positive in neoplastic astrocytic processes. (20X) C = MIB labelling with high proliferative activity of >18% (40X).

Atypical Teratoid / Rhabdoid Tumour.
A= MRI showed large space occupying mass in anterior half of left haemicranial fossa.
B= INI showed loss of nuclear expression in tumour cells. (40X)

Haemangioblastoma.
A= MRI showed 4.8x 8.4x 3.4cm cystic lesion in the left cerebellum. B= Reticulin stain positive in the reticular fibres of the blood vessels (40X). C= CD34 positive in the endothelial cells of the thin walled blood vessels. (40X).

Malignant Peripheral Nerve Sheath Tumour.
A= Vimentin showed strong positivity in the neoplastic spindle cells (40X). B= S-100 positive (20X).

Langerhans Cell Histiocytosis.
A= Soft tissue swelling in left parieto occipital region in MRI. B = CD1a showing positivity in Langerhans cells (40X).

Secretory Meningioma.
A=MRI showing extradural mass measuring 2.5 x 3.1mm right frontotemporal region. B=CEA positive in hyaline globules (40X).

Anaplastic Ganglioglioma.
A= MRI showing extra axial lesion, centered on greater wing of sphenoid bone with enhancing solid mural nodule & cystic areas with minimal peri-lesional oedema. B= GFAP positive in neoplastic astrocytes (40X). C= S-100 positive in the mature ganglion cells (40X).

Metastatic Adenocarcinoma.
A=Coin shaped lesion in right frontal lobe with diffuse perifocal oedema in CT scan. B= PAS positive (100X). C= TTF-1 positive in the tumour cell nuclei (20X).
Among the 12 non neoplastic lesions, cystic lesions and cerebral abscess constituted of six (50%) and four (33.4%) cases respectively. A single case each of infarct and giant cell reparative granuloma were also noted. The highest number of non neoplastic lesions was encountered in third decade, with a male predominance [Table/Fig-2].
Discussion
The analysis shows that these 62 cases of ICSOLs share several features common with other published series. Many reports have suggested that both incidence and pattern of intracranial neoplasms are subject to considerable geographic and racial variation [7]. Although data about the spectrum of CNS tumours in our population is available from centers across our country, each one has its own limitation in terms of the study population; therefore a single set of results cannot be applied uniformly across the board. Keeping track of the change in trends of various diseases is essential to bring about timely improvements in clinical practices [8].
In the present study, brain tumours occurred mostly during the fourth decade of life which was consistent with most series reported from Asian countries [2]. The percentage of paediatric brain lesions occurring below the age of 20 years, in the present series was 32.2%, which was comparatively higher than other observations of Rathod et al., (21.3%), Butt et al., (18%), Kothari et al., (16%) and 11% in Mahmood et al., [2,9–11]. However, other series have reported the percentage of paediatric brain tumours to be 8% in Germany, 10 % in the United States 18.6% in china and 28.4% in Thailand. These figures seem to be related to the size of the paediatric population in each country. The most common tumours in paediatric age group were astrocytomas, followed by embryonal tumours in line with other published reports [9].
The male to female ratio of 1:1.14 in the present series of 62 cases showed that there is female predominance. This trend is similar to that observed by Kothari et al., [10] but a male preponderance was present in non neoplastic lesions which correlated well with the observations of Butt et al., [9].
Tumours of the neuroepithelial origin 27 (56.3%) cases were the most frequent type of intracranial neoplasms among all primary CNS tumours in the present series which was in agreement with all other observations [2,9,10,12] as shown in [Table/Fig-13].
Comparison of Histological Type of CNS Neoplasms.
| Sl. No | Lesions | Buttet al.,(2005)[9] | Rathodet al.,(2010) [2] | Guptaet al.,(2012) [12] | Kothariet al.,(2014) [10] | Present study |
|---|
| 1 | Neuroepithelial tumours | 41(51.8%) | 17(68%) | 57(54.8%) | 25(53.2%) | 27(56.3%) |
| 2 | Meningiomas | 23(29.1%) | 05(20%) | 21(20.2%) | 11(23.4 %) | 06(12.5%) |
| 3 | Haemangioblastoma | | | | 03(6.4%) | 02(4.2%) |
| 4 | Neurilemmoma | 11(13.9%) | 01(4%) | 12(11.5%) | 03(6.4%) | 07(14.6%) |
| 5 | Pituitary adenomas | 02(2.5%) | 02(8%) | 12(11.5%) | 04(8.5%) | 01 |
| 6 | Germ cell tumours | 01(1.3%) | - | - | - | 01 |
| 7 | Histiocytic tumours | - | - | - | - | 02(4.2%) |
| 8 | MPNST | - | - | - | - | 01 |
| 9 | Epidermoid tumour | - | - | - | | 01 |
| 10 | Lymphoma(NHL) | 01(1.3%) | - | 02(2%) | 01(2.1%) | - |
| Total | 79 | 25 | 104 | 47 | 48 |
As regards to age distribution of tumours, neuroepithelial tumours occurred at a significantly younger age in the present study. These findings were comparable to that of Ejaz Butt et al., who also reported that majority of the neuroepithelial tumours were found in a younger age [9].
Of the 63 tumours diagnosed by Anand et al., gliomas comprised the largest category with 52.4% among the primary tumours as compared to 50% in the present study [13], whereas Chawla et al., Butt and Kothari comprised of 38.7%,47% and 62.3% of gliomas in their respective studies [5,9,10].
In the present study, out of 24 gliomas majority were Grade I constituting of 9 cases (37.5%) followed by 8 cases of Grade IV accounting for 33.3% according to WHO grading, whereas in the study of Kothari et al., majority were Grade IV with 11 cases (55%) out of 20 gliomas followed by Grade II with 7 cases (35%) [10]. Hence grading of gliomas is extremely important to predict their aggressiveness and prognosis in deciding further plan of treatment [10].
The relative frequency of meningiomas in this series was 12.5% of all primary tumours. This view is supported by other studies which reported 18% (Chawla et al.,) 11% (Kalyani D et al.,) in a series of 47 and 77 primary intracranial tumours [5,14]. Among the primary tumours, 16.7% were tumour of meninges with Grade I forming the majority which accounted for 7 cases (87.5%) according to WHO grading in the present study. This was in agreement with Kalyani D et al., who also reported Grade I as majority with 6 cases (75%) [14]. A female preponderance was seen in meningiomas as in all other studies [9,10,12,15], indicating that the growth of meningiomas is subject to hormonal influence as reported by Kothari et al., [10].
Headache was the most common presenting symptom in this series which is supported by the findings of all other studies [2,10–12]. Though a small number of cystic lesions were encountered in the present study an attempt has been made to compare with other studies [2,9,10,14], which revealed epidermoid cyst to be the predominant cystic lesion but the present study reported equal number of epidermoid and arachnoid cyts accounting for 25% each among the non-neoplastic lesions. Female preponderance was seen among epidermoid cyst in the present study. This was in agreement with Rathod et al., and Butt et al., who also observed similar features [2,9].
Unlike other studies, among the 12 cases of non-neoplastic intracranial masses the present study encountered 4 cases of cerebral abscess after the cystic lesions with male predominance. Except Butt et al., [9], all other studies also revealed male predominance among the cerebral abscess [2,11,13].
IHC plays an important role in the confirmation of diagnosis. GFAP is currently being employed to assist in the diagnosis of human brain tumours. Positive reaction to GFA protein has been demonstrated in astrocytomas and astrocytic cells of mixed gliomas, subependymal giant cell astrocytoma and gliosarcoma [5].
Limitation
Few immunohistochemistry markers like TP53, INI1, MIB 1, CD 1a, CEA, TTF1 could not be carried out at our centre and specimen was sent to higher centre for confirmation of diagnosis.
The specimens for this study were obtained from our newly set up Neurosurgery department, which drains a restricted population from the North Karnataka region.
Conclusion
This study has highlighted the relative frequency of different intracranial space occupying lesions in Kalaburagi region. The availability of clinical information and neuro imaging techniques like CT scan and MRI are of considerable importance for final histopathologic diagnosis. Preoperative diagnosis was compared with postoperative pathological diagnosis. The utility of IHC is emphasized in the present study. GFAP is a sensitive and specific marker for glial differentiation and establishing the origin of astrocytic tumour.
The diagnosis of intracranial space occupying lesions can be challenging due to difficulty exacerbated by the small size of the specimen. Specialities of neurosurgery, neuroradiology and neuropathology must converge to diagnose the lesion accurately to aid in appropriate management for better patient care and follow-up.
M=Male, F=Female