Mummification is man’s earliest way of human tissue preservation. Good tissue specimen preparation require complete fixation [1]. As early as 400 BC, Hippocrates debated about the biological events occurring in mercury and alcohol fixatives [2]. Fixation preserves and hardens cell and tissue constituents in as close as a life-like state as possible and allows these tissues to undergo further preparative procedures without change [1]. It prevents autolysis, attack by bacteria, and change in volume and shape so that they withstand the subsequent stages of tissue processing [3]. Faults in fixation are permanent and cannot be corrected at any later stage whereas any other step after proper fixation can be reversed to refine a problem [4].
Materials and Methods
This was a comparative study which was carried out in the Department of Oral Pathology, Kothiwal Dental College and Research Center, Moradabad, U.P., India, over a period of one year from September 2012 to September 2013. The ethical clearance for the study was obtained from Institutional Ethical Committee.
Tissue sample used for the study [Table/Fig-1,2]: Goat tongue was used as the tissue sample which was harvested from a butcher shop. Total of eight tongue specimens were taken for each cycle and each specimen was further divided as to be used at five different time intervals.
Collection of specimen (goat tongue).
Immediate transfer of specimen.
Fixatives selected: 10% Buffered Formalin, Carnoy’s Solution, Absolute Ethyl Alcohol and Bouin’s Fluid.
Methodology: The present in-vitro study was conducted over a period of one year. Fresh tissue specimens (goat tongue) were obtained from a slaughter house and were immediately transferred to the fixatives. No animal was harmed for the purpose of the study. Each tongue tissue was collected and it’s anterior and posterior-third was discarded. The middle third portion of the tongue was retained and was used for the study purpose. The tissue was grossed into 10 equal parts (1cm each) and were kept in above mentioned fixatives for five different time intervals of 6, 12, 18, 24 and 30 hours respectively. After fixation, normal tissue processing steps were carried out followed by sectioning and staining [Table/Fig-3,4]. These fixation and processing steps were repeated 20 times. All the data obtained was compared and statistically analyzed.
| 70% Alcohol | ½ hours |
| 95% Alcohol | ½ hours |
| 100% Alcohol | Overnight |
| Xylene I | 1 hour |
| Xylene II | 1 hour |
| Xylene III | 1 hour |
| Wax bath | 4 hours |
| Paraffin blocks preparation followed by sectioning by microtome and then staining. |
| 100% Alcohol | Slide was dipped for 1 minute |
| 90% Alcohol | Slide was dipped for 1 minute |
| 80% Alcohol | Slide was dipped for 1 minute |
| Tap water | 1 dip |
| Hematoxylin | 3 minutes |
| Tap water | 1 dip |
| 1% Acid Alcohol | 1 dip |
| Blueing | 15 minutes |
| Eosin | 2 minutes |
| 80% Alcohol | 1.5 minute |
| 90% Alcohol | 1.5 minute |
| 100% Alcohol | 2 minute |
| Xylene | After drying, clearing for 15 minutes |
| DPX | Mounting |
Scoring: The data was statistically analyzed under following headings:
Sectioning criteria: It was evaluated by the presence and absence of two factors; Hard to cut and Crumble.
Staining criteria: It was evaluated under light microscope at 10X magnification by scoring the slides from 0-5 (score-3 was kept as minimum score for acceptable result) by three independent observers under two parameters; Nuclear staining and Cytoplasmic staining.
Microscopic details: It was evaluated under light microscope at 10X magnification by scoring the slides from 0-5 (score-3 was kept as minimum score for acceptable result) by three independent observers under two parameters; nuclear and cellular shrinkage, nuclear and cellular dissolution/distortion of cellular components.
Score 0 – 5, indicates:
0 (very poor)
1 (Poor)
2 (Average)
3 (Good)
4 (Very good)
5 (Excellent)
Statistical Analysis
Comparison of various parameters with standard parameter was analyzed by applying appropriate statistical methods. The descriptive results were presented as median. Comparison of sectioning criteria was carried out by using fisher’s exact test with p-value < 0.05 as significant. Comparison of staining criteria and microscopic details was carried out using Mann-Whitney U test with p-value < 0.05 as significant. Statistical analysis was conducted using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
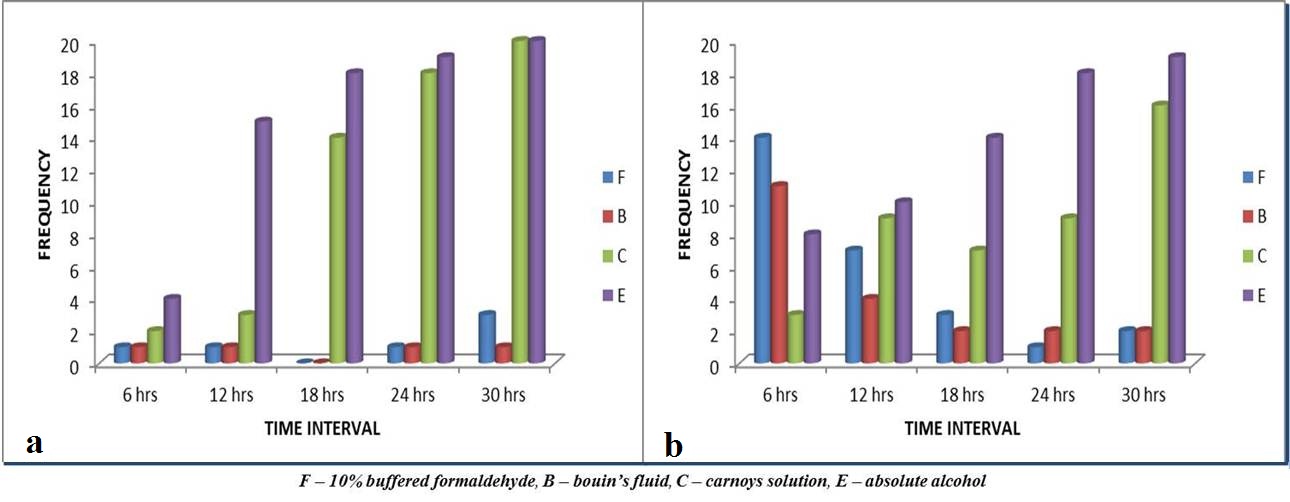
Hard to cut: As time interval increases, hard to cut and crumbling frequency of specimens in absolute alcohol and Carnoy’s solution increases [Table/Fig-5a,b]. Twelve hours onwards specimens in absolute alcohol became hard to cut whereas from 18 hours onwards specimens in Carnoy’s solution became hard to cut during sectioning by microtome, which were statistically significant [Table/Fig-6].
Comparison of sectioning criteria. Graphs showing hard to cut (a) and crumbling (b) frequency during sectioning.
Sectioning criteria: Overall comparison of different fixatives at different time intervals with the standard fixation criteria using fisher’s exact test.
| Time Interval | 6 hours | 12 hours | 18 hours | 24 hours | 30 hours |
|---|
| Fixatives | Hard to cut | Crumble | Hard to cut | Crumble | Hard to cut | Crumble | Hard to cut | Crumble | Hard to cut | Crumble |
|---|
| Carnoy’s | 1 | 0.605 | 0.605 | 0.0084 | < 0.001 | 0.043 | < 0.001 | 0.008 | < 0.001 | < 0.001 |
| Formalin | 1 | < 0.001 | 1 | 0.008 | 1 | 0.605 | 1 | 1 | 0.605 | 1 |
| Bouin’s | 1 | < 0.001 | 1 | 0.605 | 1 | 0.341 | 1 | 0.605 | 1 | 1 |
| Ethanol | 0.341 | 0.019 | < 0.001 | 0.003 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Crumbling: The specimens in 10% buffered formalin and Bouin’s fluid showed maximum crumbling at 6 hours (p<0.001) whereas specimen in Carnoy’s solution showed minimum crumbling. As the time increased specimens in absolute alcohol and Carnoy’s solution showed maximum crumbling frequency (p<0.001) and specimens in 10% buffered formaldehyde and Bouin’s showed the least [Table/Fig-5b,6].
Nuclear staining: It was seen that the specimens in absolute alcohol and Carnoy’s solution showed good staining results at 6 hours and decreased in quality thereafter whereas those in 10% formaldehyde and bouin’s fluid showed excellent results at 24 hours as in comparison to the earlier time intervals [Table/Fig-7].
Staining and microscopic details: Overall histologic score and comparison of different fixatives at different time intervals with the standard fixation criteria.
| Parameters(Median Score) | Fixatives | 6 hours | 12 hours | 18 hours | 24 hours | 30 hours |
|---|
| N | Cy | N | Cy | N | Cy | N | Cy | N | Cy |
|---|
| Staining | Carnoy’s | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Formalin | 2 | 2 | 3 | 2 | 3 | 3 | 5 (1) * | 5(1) * | 5(0.799) * | 4(0.289) * |
| Bouin’s | 1 | 2 | 2 | 2 | 3 | 3 | 4 | 4 | 3 | 3 |
| Ethanol | 3 | 3 | 3 | 2 | 2 | 1 | 2 | 1 | 1 | 1 |
| N | C | N | C | N | C | N | C | N | C |
| Shrinkage | Carnoy’s | 4 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 2 | 0 |
| Formalin | 2 | 2 | 2 | 3 | 4 | 3 | 5(1) * | 5(1) * | 5(0.289) * | 5(0.602) * |
| Bouin’s | 2 | 3 | 3 | 3 | 4 | 3 | 4 | 4 | 5(1) * | 4 |
| Ethanol | 4 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 0 |
| Swelling | All Fixatives | Not Applicable |
| Distortion and Dissolution | Carnoy’s | 3 | 3 | 3 | 2 | 3 | 2 | 2 | 1 | 2 | 1 |
| Formalin | 2 | 2 | 3 | 3 | 3 | 3 | 5(1) * | 5(1) * | 5(1) * | 5(1) * |
| Bouin’s | 2 | 2 | 2 | 2 | 3 | 3 | 4 | 4 | 4(0.062) * | 4 |
| Ethanol | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
p < 0.001 for all except for (*), Mann-Whitney U test, N-nuclear, Cy-cytoplasmic, C-cellular
Cytoplasmic staining: Specimens in absolute alcohol and Carnoy’s solution showed good results at 6 hours and those in 10% formaldehyde and Bouin’s fluid showed excellent results at 24 hours [Table/Fig-7].
Nuclear and cellular shrinkage: It was seen that ethanol based fixatives showed high shrinkage at extended time intervals (lesser the score poorer the quality of slide) [Table/Fig-7].
Nuclear and cellular distortion/dissolution: Specimens fixed in 10% formaldehyde and Bouin’s showed high nuclear and cellular distortion/dissolution at 6 hour time interval where as those in Carnoy’s and absolute alcohol showed high nuclear and cellular distortion/dissolution at 30 hour time interval [Table/Fig-7].
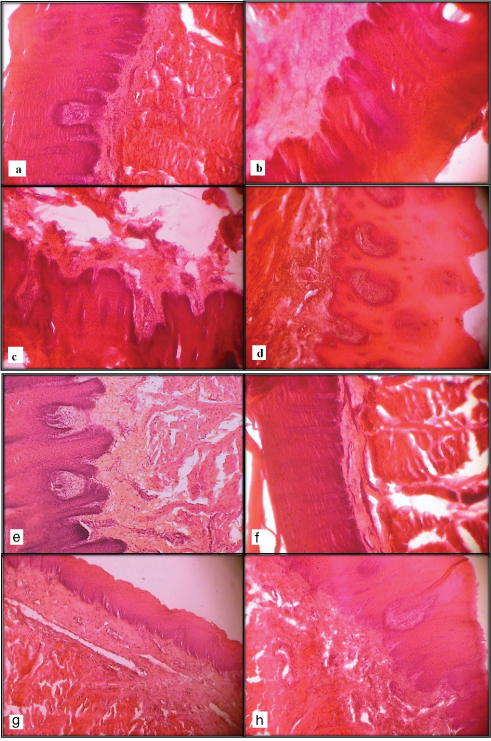
The effect of various fixatives at different time intervals is shown in [Table/Fiig-8a-h].
Photomicrographs (10X) of tissue kept in Carnoy’s fluid for 6 hours (a) and 30 hours (b), in Absolute alcohol for 6 hours (c) and 18 hours (d). Photomicrographs (10X) of tissue kept in 10% buffered formaldehyde for 24 hours (a) and 6 hours (b), in Bouin’s solution for 24 hours (c) and 12 hours (d).
Discussion
Fixation is a critical step in tissue preparation and errors in fixation can cause irreversible damage to the tissue specimen. No matter how much care is subsequently taken in tissue processing, microtomy and staining, the morphological and histochemical information obtainable from the specimen will be compromised. Fixation aims to prevent putrefaction and autolysis which starts with the discontinuation of the blood supply to the tissue. It is therefore important that the principles and practice of tissue fixation are applied to obtain quality slides. Buffered formalin 10% is the universal fixative for routine paraffin embedded sections. But, the use of other fixatives is also increasing due to their easy usage and availability.
Absolute alcohol has very little place in the routine fixation of tissue for histopathology [5]. But it’s easy availability and fast fixation time prompts us to choose this fixative. It is used to fix blood films and smears [10]. If ethanol is used alone, it causes collapse of protein structures, thus exposure must be limited to 8 hours or less. Addition of water causes considerable swelling of proteins and subsequent shrinkage in absolute alcohol. Hence, the ratio of fixative and tissue should not be less than 20:1 [11]. It penetrates rather slowly and tends to hardens tissue after long exposure as seen in the present study from 12 hours onwards [10] [Table/Fig-5a,6].
Absolute alcohol in combination with chloroform and glacial acetic acid is called as Carnoy’s fluid which prevents the considerable shrinkage found in alcohol fixed material [11]. A review of the chemical literature shows that the components of Carnoy’s solution; ethanol, chloroform and acetic acid – can interact by hydrogen bond formation with each other and with various groups in tissues, which seems to stabilize tissue structures. These components are commonly available in pathology laboratories and thus Carnoy’s fluid can be easily prepared fresh. Complete fixation usually requires 1-2 hours prior to processing. It causes considerable shrinkage and destruction and dissolution of most cytoplasmic elements [10], as seen in the results at 30 hour time interval [Table/Fig-8b]. If nucleic acids are to be studied in paraffin sections, then Carnoy’s fixative is recommended [5]. Carnoy’s fluid is suitable for a many conventional and special staining techniques and is commonly used for histochemical studies of fibrous proteins and associated carbohydrates [12]. But its use for the identification of nucleic acid and proteins for diagnostic purposes is limited due to lack of advances in specific methods available, immunohistochemistry, in-situ hybridization and proteomics [5].
The main components of Bouin’s fluid are: picric acid, glacial acetic acid and formalin, which are commonly available in pathology laboratories and thus this fluid can be easily prepared when needed. Bouin’s fluid penetrates tissues rapidly and can cause little shrinkage. Tissues fixed in it are best suitable for staining by trichrome methods. It can be used to demonstrate glycogen [10]. It requires 24 hours fixation prior to processing cycles [5]. Picric acid is a slow penetrating fixative that precipitates proteins by forming salts (picrates) with basic proteins but causes tissue shrinkage. Glacial acetic acid tends to bring about swelling, which partially counteracts the shrinkage from the picric acid [13]. The presence of picric acid in this fixative is associated with bright yellow staining of the tissue and thus requires tedious rinses with alcohol in order to remove the excess yellow colour. Bouin’s fixative is a non-coagulant picrate solution, but it is not suitable as a preservative. Tissues cannot be stored for extended periods because of shrinkage induced by picric acid which forms the major component of the Bouin’s fixative. Bouin’s provides excellent preservation of nuclear details allowing a more accurate pathological assessment which can be important in determining the difference between inflammation and malignancy. Tissues which are small and delicate are fixed in Bouin’s solution because of better preservation of nuclear architecture by this method as was also seen in the present study at 24 hours [14] [Table/Fig-8g].
Saturated solution of 40% formaldehyde gas in water is termed as formalin [10]. It is an inexpensive, commonly available fixative that causes least tissue shrinkage or distortion of cellular structure [Table/Fig-8e]. Formalin 10% is routinely used as a fixative and has an extensive use in tissue fixation for diagnostic purposes. Methanol 10% in formalin is used as a preservative. It is a 2-phase fixative in which alcohol fixation phase causes tissue dehydration and hardening followed by cross-linking phase mediated by aldehyde. A longer period of formalin storage causes formation of formic acid due to oxidation which can precipitate formalin pigments in a tissue section due to reaction with blood. Hence, the use of freshly prepared buffered formalin is preferred [15].
In the present study, at any time period specimens in absolute alcohol were hardest to cut followed by Carnoy’s, 10% buffered formalin and Bouin’s which was easiest to cut. This finding was similar to the findings in the study conducted by Stickland NC [16]. Above findings could be explained by the fact that alcohol fixed tissues were sometimes slightly brittle as compared with formalin fixed tissue as reported by Bostwick DG et al., [17] also with extended ethanol fixation, tissue can also be over-hardened as described by Troiano NW et al., [8]. Excessive hard tissue is a known cause of ‘chatter’ as stated by Choi JH et al., [18].
At 30 hours, specimens in 10% formaldehyde showed slightly higher frequency of hard to cut criteria as compared to the specimens fixed for the lesser time in the same fixative. This could be due to the fact that with extended formalin fixation, cross-linking of the proteins continue to occur long after the fixative has penetrated the tissue and this continued cross linking may cause the tissue to harden, resulting in over-hardened tissue as reported in the study conducted by Troiano NW et al., [8]. At 6 hours, for specimens in 10% buffered formalin and Bouin’s fluid showed highest crumbling with former even higher. This could be due to incomplete fixation as when formaldehyde is used as a fixative in aqueous solution, at least 24 hours at room temperature or 16 hours at 37°C are required for the reaction to reach equilibrium as described by Fox CH et al., [7] and due to incomplete fixation tissue components can separate easily on the water-bath during microtomy as reported by Freida L. Carson [4].
At 6 hour time intervals specimens in the Carnoy’s solution may be adequately fixed and showed least crumbling. With increasing period of time, specimens in absolute alcohol and Carnoy’s solution showed increased crumbling which could be due to over-hardened tissue as previously discussed.
Results under nuclear and cytoplasmic staining criteria [Table/Fig-3,4], were similar as attained by Cox ML et al., [19]. In contrast to the above findings Bultitude MF et al., [14] found that there was better preservation of nuclear details in the specimens fixed in Bouin’s solution than formalin fixation. Also at 24 hours, score of nuclear and cellular details for the specimens in 10% buffered formalin is much higher than that of absolute alcohol. This is similar to the finding described by Poul Prento and Hans Lyon [20]. Specimens in ethanol showed decrease of nuclear staining, cytoplasmic changes and shrinkage over time. This finding is also similar to the findings of Yoko Matsuda et al., in which 99% ethanol fixation showed decrease of nuclear staining as compared to 10% neutral buffered formalin [21].
From 24 to 30 hours specimens in 10% buffered formaldehyde showed the best results for nuclear staining with median score of 5 followed by specimen in Bouin’s fluid whereas specimens in absolute alcohol and Carnoy’s solution showed acceptable results at 6 hours and 12 hours respectively with median score of 3. This could be because ethanol mainly demonstrate fibrous cytoskeletal proteins whereas formalin crosslinks nucleic acids and thus produce good nuclear detail as described by Nancy W. Troiano et al., [8]. Ethanol fixation affords excellent preservation of cellular details, but at the expense of slightly higher cell shrinkage than occurs with formalin as described by H Battifora and M Kopinski [22].
In contrast to the above observations, tissues fixed in alcohol can offer superior morphology was noted by Gillespie et al., who ranked 95% ethanol as having superior morphology to 10% Neutral Buffered Formalin (NBF) based on nuclear morphology, cellular morphology, tissue architecture, and staining characteristics described in the study conducted by Melissa L. Cox et al., [19].
Poor staining quality in case of tissues fixed in Bouin’s fluid at 12 hours [Table/Fig-8h] and 10% buffered formaldehyde at 6 hours [Table/Fig-8f] could be due to poor fixation. As described by Stephen M. Hewitt et al., [23] poor fixation leads to incomplete dehydration as residual water will not be replaced by paraffin, thus making tissue susceptible to degradation [23]. Inadequately dehydrated tissue will be partially unstained as stated by Elizabeth McInnes [24]. Also, poor staining quality in case of tissues fixed in Carnoy’s solution at 30 hours [Table/Fig-8b] and absolute alcohol at 18 hours [Table/Fig-8d] could be due to the fact that tissue that is over-fixed is less penetrable to stain, resulting in poorer staining quality as described by Nancy W. Troiano et al., [8].
Specimens in 10% formaldehyde at 6 hours and Bouin’s fluid at 12 hours showed poor nuclear/cellular morphology and distortion [Table/Fig-8f-h]. This could be due to incomplete fixation as described by Freida L. Carson [4] in which he stated that nuclei may be muddy/ smudgy and tissue morphology is not well maintained. Paul Prento and Hans Lyon [20] also stated that for several fixatives like ethanol, histo choice, deterioration of cell structure and extensive vacuolization of the cytoplasm indicated delayed or perhaps lack of penetration.
As the time interval increases above 6 hours, specimens in Carnoys solution and absolute alcohol showed poor nuclear/cellular morphology and distortion [Table/Fig-8b-d]. This could also be explained by the fact that cell shrinkage due to extended ethanol fixation can result in cell morphology alteration and tissue can also become over-hardened as described by Nancy W. Troiano et al., [8].
It was found that for adequate fixation; minimum time required for the tissues to be kept in 10% buffered formalin and Bouin’s solution is 18 hours. Tissues could be kept for a maximum time of 6 hours in Carnoy’s fluid and absolute alcohol [Table/Fig-8a-c]. Carnoy’s fluid showed the fastest tissue fixation with good results and thus could be used in case diagnosis is urgently required.
Buffered formaldehyde 10% fixation for 24 hours continues to be the best fixation method. But as an alternative to formalin fixation or as a second option 24 hours Bouin’s fixation gives nearly equivalent score to formalin and can be used as an alternate fixative followed by Carnoy’s and absolute alcohol fixation at 6 hours giving acceptable results [Table/Fig-7]. To the best of our knowledge, present study is first of its kind where tissues in four different fixatives were kept for 5 different time intervals (6, 12, 18, 24 and 30 hour) and were compared with each other.
Limitation
Being an animal study, further studies are required with variable and larger sample size with added criterias and also with humans pathological tissues to further authenticate the observation and conclusion of the present study for clinical use.
Conclusion
Use of 10% buffered formaldehyde should be continued as a routine fixative but owing to its potential human health hazards like its noxious vapor may cause allergic reactions, affects respiratory epithelium and known to be a probable human carcinogen other fixatives can be used as an alternative owing to their less toxicity, easy availabilities and preparation in the laboratories or non-availability of a required fixative. Pathologist should be accustomed to histologic and morphologic changes of underfixed and overfixed tissue with different fixatives which can effect its diagnostic value.
p < 0.001 for all except for (*), Mann-Whitney U test, N-nuclear, Cy-cytoplasmic, C-cellular