Ameloblastomas are common odontogenic tumours that are benign and locally aggressive. Histopathologically, the tumor exhibits significant diversity with common and rare variants. Here, we report an unusual variant of a common odontogenic tumour in the mandibular posterior region on the right side in a 44-year old male patient. This is the sixth case of Papilliferous Keratoameloblastoma (PKA) to be reported in the English literature till date. More case reports are vital to determine the clinical, radiological, histopathological and behavioural aspects of this extremely rare histological type of ameloblastoma. This tumour awaits re-inclusion as a distinct entity in the future classifications of the WHO Classification of head and neck tumours upon further case accrual.
Case Report
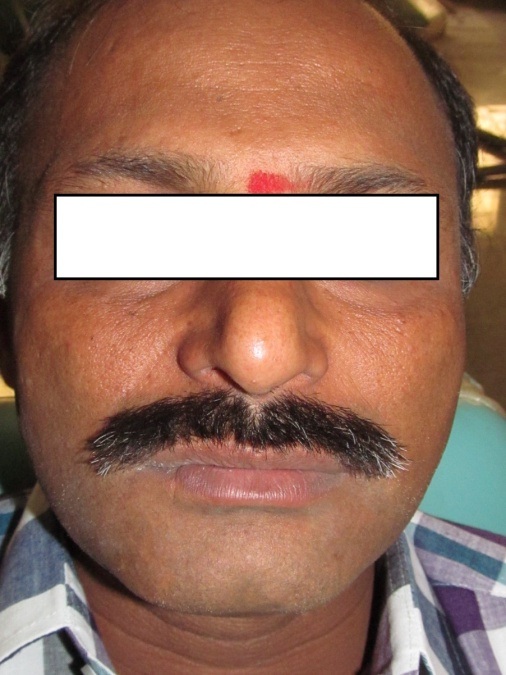
A 44-year-old Indian male visited the outpatient department with a chief complaint of a swelling on the lower right back tooth region since six months. The lesion gradually increased to the present size of 3cm X 4cm in diameter. Intermittent pain was associated with the swelling with gradual mobility of the teeth. Extraoral examination revealed a mild ill-defined swelling at the right corner of the mouth without any loss of facial asymmetry. The overlying skin appeared normal with no rise in the local temperature [Table/Fig-1].
Clinical photograph showing ill-defined swelling at the right corner of the mouth with normal overlying skin.
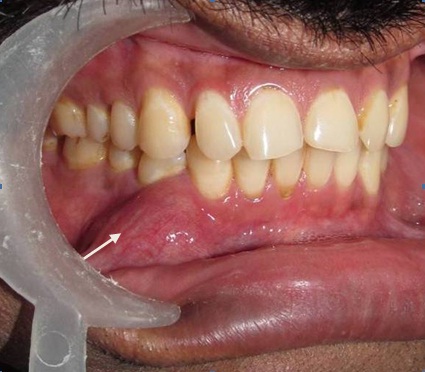
Intraoral examination revealed a solitary swelling on the right alveolar mucosa measuring approximately 3.5cm X 3.0cm in diameter, ovoid in shape, extending from tooth 43 to 45 region with obliteration of the buccal vestibule. There was no tenderness on palpation or any signs of discharge. The lesion was hard in consistency and margins were diffuse. The overlying mucosa appeared normal [Table/Fig-2]. There was no associated cervical lymphadenopathy. The patient’s periodontal status was good with presence of the full complement of permanent teeth. Grade I mobility was seen in 43. Grade III mobility and deep periodontal pocket was noticed in 44.
Clinical photograph showing swelling in right buccal mucosa extending from 43 to 45 region with obliteration of buccal vestibule.
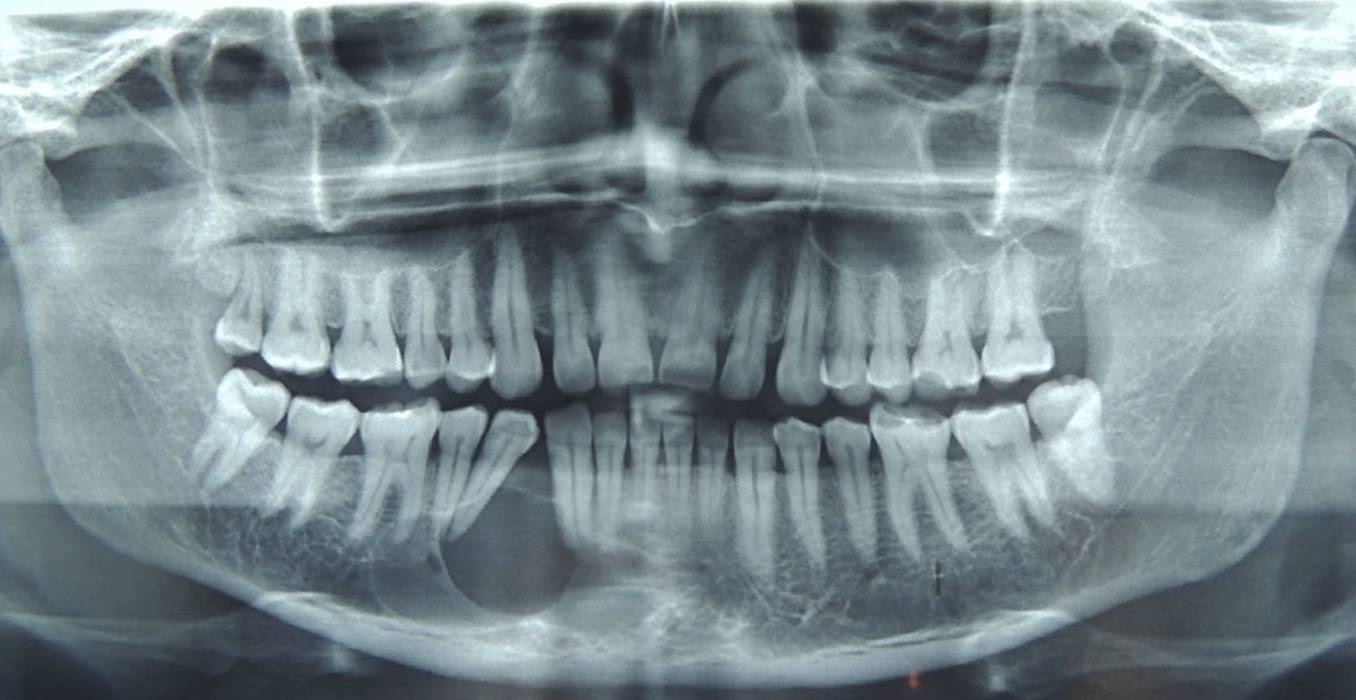
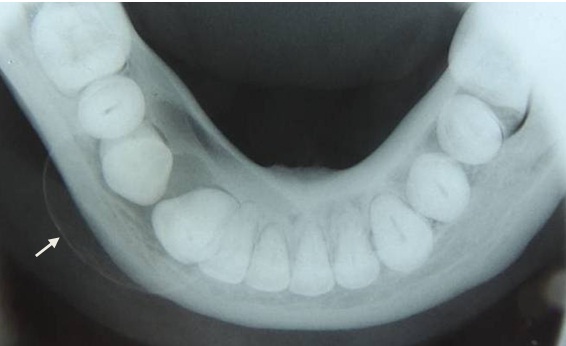
Orthopantomograph (OPG) revealed a predominantly well-defined unilocular radiolucent lesion involving the periapical region of mesial root of 46, 45, extending between the roots of 44 and 43; and involving the periapical region of 42 and 41, 44 was distally drifted [Table/Fig-3]. Occlusal radiograph showed buccal cortical expansion and thinning of lingual cortex at the lesional area [Table/Fig-4].
Orthopantomograph showing a well defined unilocular radiolucent lesion in the periapical region extending from 43 to 46.
Occlusal radiograph showing buccal cortical expansion and thinning of lingual cortex.
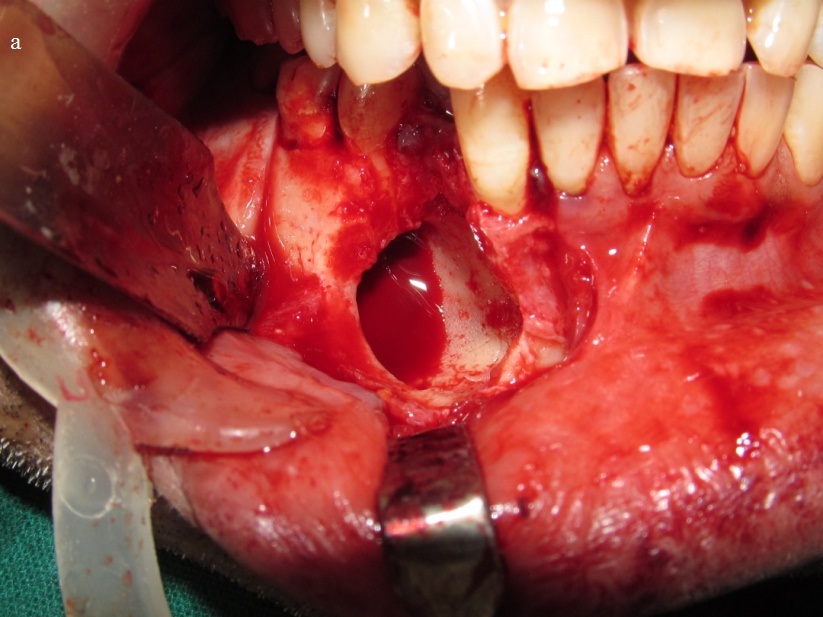
On aspiration serosanguineous fluid was obtained. The smear showed RBCs with few inflammatory cells comprising of neutrophils and lymphocytes. The provisional diagnosis was lateral periodontal cyst with clinical differentials of odontogenic keratocyst, glandular odontogenic cyst, unicystic ameloblastoma, aneurysmal bone cyst and cystic adenomatoid odontogenic tumour. The lesion was then excised in toto along with extraction of 44 under local anaesthesia and submitted for histopathological examination [Table/Fig-5a,5b].
Intra-operative clinical image showing cystic cavity in the mandible.
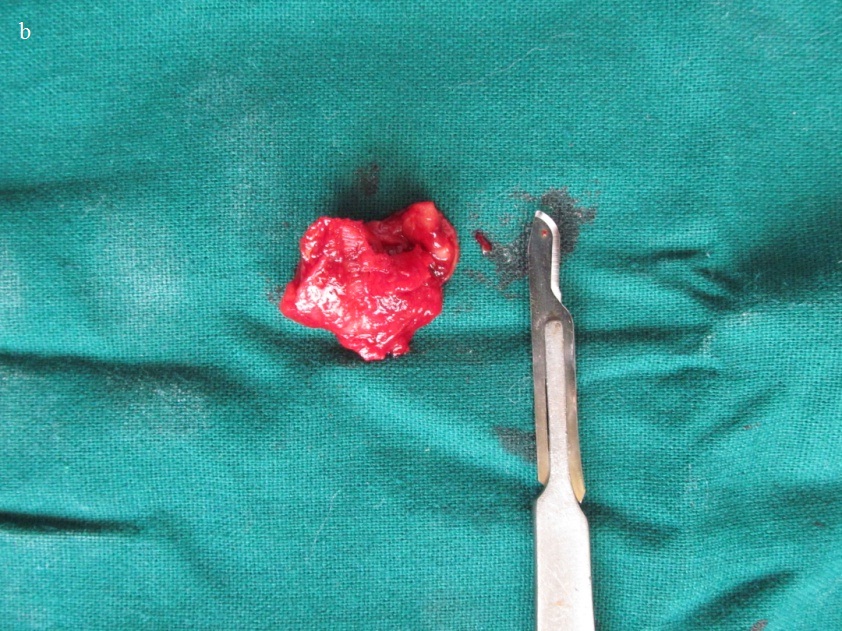
Post-operative image of the excised lesion.
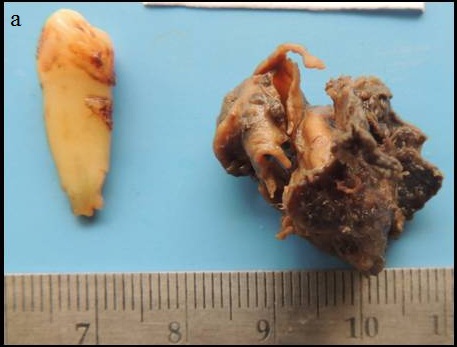
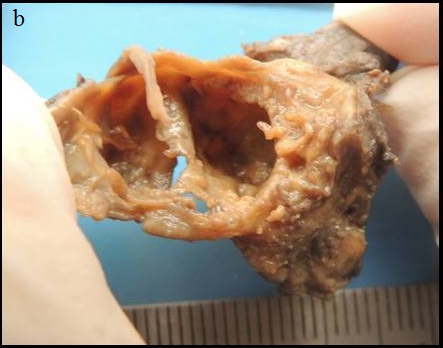
On macroscopic examination, the soft tissue received was irregular in shape, brown in colour, measuring 2.8cm X 2.0cm with firm to gritty consistency and with irregular surface [Table/Fig-6a,6b]. There was a cystic cavity lined by irregularly thickened cystic wall with numerous luminal proliferations. Solid areas were seen associated with the cystic lesion.
Macroscopic image showing soft tissue bit that is irregular in shape and surface, brown in colour, measuring 2.8cm X 2.0 cm with extracted 44.
Macroscopic image showing the cystic nature of the soft tissue specimen.
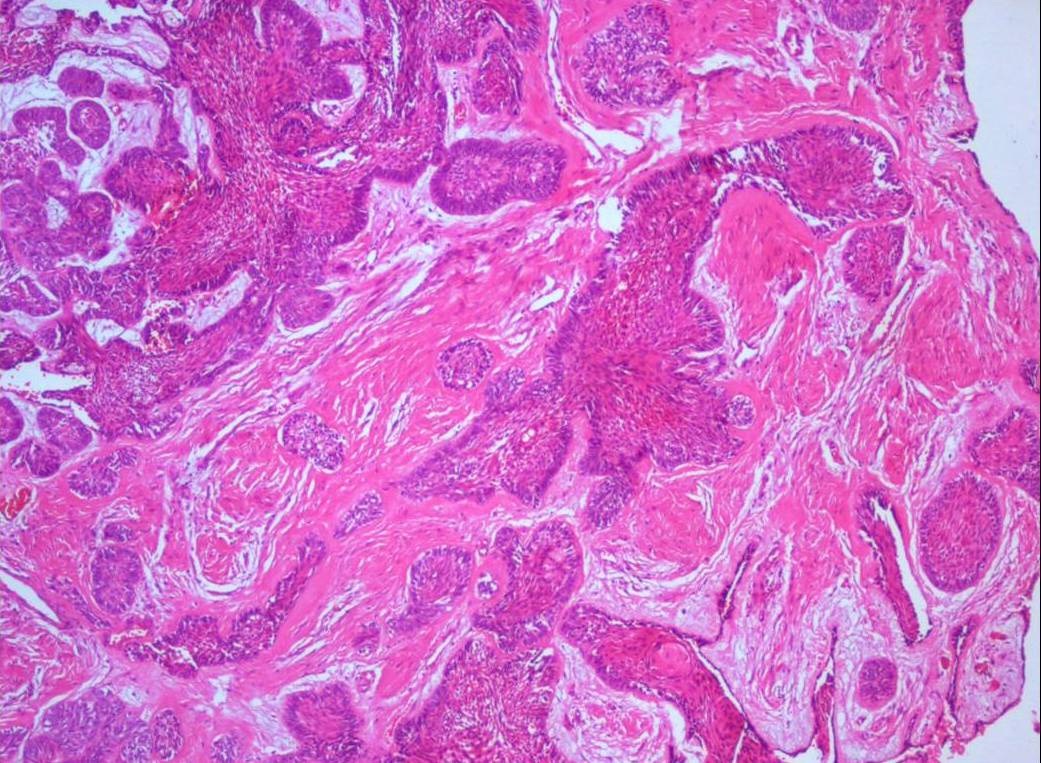
Histopathological examination of formalin fixed, hematoxylin and eosin stained sections revealed a cystic lining composed of odontogenic epithelium exhibiting predominant squamous metaplasia [Table/Fig-7]. Mural proliferations of this lining were evident in the connective tissue stroma presenting predominantly a plexiform growth pattern [Table/Fig-8]. The peripheral cells were cuboidal to tall, columnar ameloblast-like cells, with palisading nucleus being polarized away from the basement membrane. Hyperplasia of the peripheral cells was a recurring feature observed. The central cells were angular resembling stellate reticulum like cells [Table/Fig-9]. Extensive areas of squamous differentiation were seen to a point of keratin pearl formation replacing the stellate reticulum like cells [Table/Fig-10]. Sheets of squamous cells in the connective tissue stroma were evident in few areas along with extruded keratin which could be found eliciting a chronic inflammatory response. Numerous papillary extrusions of odontogenic epithelium were evident; wherein the ameloblastomatous epithelium was thrown into finger like extensions protruding into the connective tissue stroma [Table/Fig-11]. The supporting stroma was mature with collagen exhibiting marked desmoplasia [Table/Fig-12] and infiltrated with mild chronic inflammatory cells [Table/Fig-8]. A diagnosis of solid multicystic ameloblastoma of the papilliferous keratinizing variant of ameloblastoma was made based on all the above observations.
Photomicrograph showing a cystic lining composed of odontogenic epithelium. H&E X200.
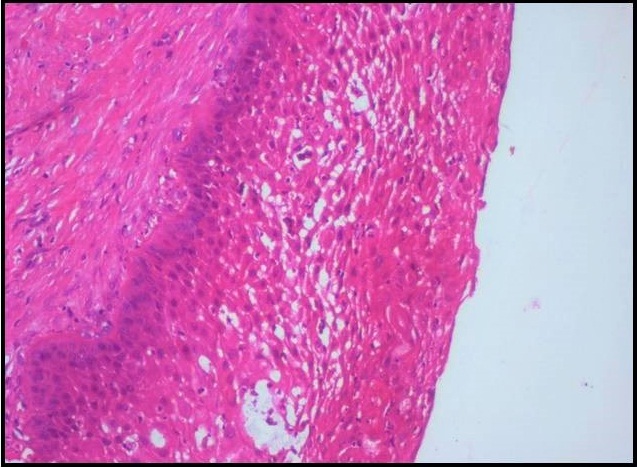
Photomicrograph showing predominantly a plexiform growth pattern with extensive squamous metaplasia in the central cells. The supporting stroma shows chronic inflammatory cells, H&E X40.
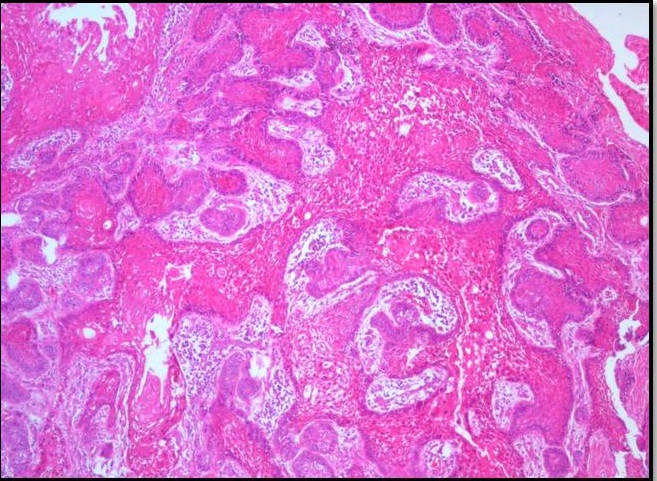
Photomicrograph showing odontogenic epithelium with peripheral tall ameloblasts-like cells exhibiting palisaded nucleus and central cells resembling stellate reticulum-like cells H&E X400.
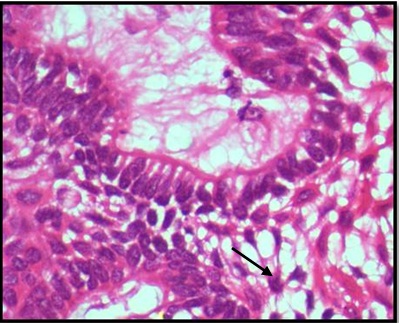
Photomicrograph showing extensive squamous differentiated areas to a point of keratin pearl formation. H&E X100.
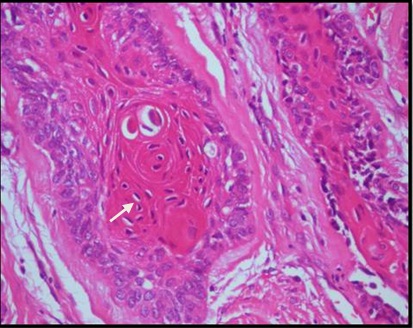
Photomicrograph showing numerous papillary extrusions of odontogenic epithelium. H&E X40.
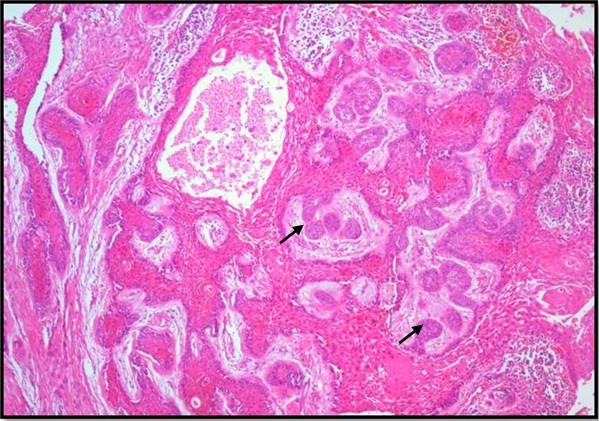
Photomicrograph showing supporting mature stroma with collagen exhibiting marked desmoplasia, H&E X40.
The follow-up of the patient was uneventful for a period of one year after which the patient was lost for follow-up.
Discussion
Solid Multicystic Ameloblastoma (SMA) is a benign epithelial tumor of odontogenic origin with a tendency to recur and is of aggressive behaviour. Solid/multicystic, peripheral, desmoplastic and unicystic ameloblastomas are clinical types of ameloblastoma [1]. Most common histologic variants of ameloblastoma are follicular and plexiform types, whereas the rare types are keratoameoblastoma and Papilliferous Keratoameloblastoma (PKA). Till date 17 cases of keratoameloblastomas are reported with the latest case being reported in 2015 with complex histology [2]. Keratoameloblastomas reported so far have been categorized into the following four groups based on their histologic presentation: 1) Papilliferous histology 2) Simple histology 3) Simple histology with Odontogenic Keratocyst (OKC) like features and 4) Complex histology [3].
Pindborg in 1970 reported an unusual type of ameloblastoma consisting of keratinizing cysts and tumor islands with papilliferous appearance and named it as PKA [4]. Later four more cases of PKA were reported in 1991 [5], 1994 [6], 2002 [7] and 2013 [8]. The present case is the sixth one to be reported in the English literature.
PKA is essentially based on the histologic presentation of ameloblastoma. PKA is an exceedingly rare, slow growing, benign, epithelial odontogenic neoplasm which presents as a non-encapsulated and locally invasive lesion. Initial reports described this entity as exhibiting a unique histological pattern characterized by multiple epithelial cysts of varying sizes. Vast majority of the cysts are lined by a non-keratinized papilliferous epithelium and filled with necrotic desquamated epithelial cells [9].
Keratoameloblastoma with papilliferous histology presents at a mean age of 40 years with a male predilection. Our patient being 44 years of age approximately falls in a similar age group and gender. The frequent site of occurrence in reported and our case is the posterior mandible [10]. Radiographically the lesions present as multilocular radiolucent lesions, but in contrast our case had a well circumscribed unilocular radiolucency and had occurred in the tooth bearing area of the jaw. All the five cases reported are summarized below [Table/Fig-13].
Previous cases of keratoameloblastoma with papilliferous histology reported in literature.
| S.No. | Author | Age/ Gender | Clinical Site | Radiographic Findings | Treatment and Follow-up |
|---|
| 1 | Pindborg [4] | 57/F | Right mandibular body and ramus | Multilocular radiolucency | Unknown |
| 2 | Altini [5] | 76/ M | Right mandible | Multilocular radiolucency | Hemi mandibulectomy. Uneventful till 12 months |
| 3 | Norvel [6] | 26/ F | Right mandible | Lobulated radiolucency | Segmental resection. Unknown. |
| 4 | Collini [7] | 62 /M | Right ramus and condyle of mandible | Irregular radiolucency with calcification | Hemi mandibulectomy.Two local recurrences at 36 and 38 months |
| 5 | Mohanty [8] | 46 /M | Right posterior mandible | Multilocular radiolucency | Unknown |
| 6. | Present case | 44/M | Right posterior mandible | Unilocular radiolucency | Excision. Uneventful till 12 months |
The papillary nature could be explained by the loss of intercellular adherence in the surface layers, which results in desquamation and necrosis of the cells. The necrotic cells separate from the remainder of the epithelium resulting in the formation of numerous pseudopapillary structures which project into the lumina of the cystic follicles [8].
More recent reports however, have described certain distinct features like the papillary projections to be true papillary projections with a connective tissue core and surface keratinization which protruded into the stroma [9]. In these cases, the peripheral cells of the odontogenic follicles were tall, columnar and palisaded with central stellate reticulum like cells [11].
In the present case, connective tissue stroma with solid sheets, islands and strands of odontogenic epithelium with predominantly central squamous metaplasia replacing the stellate reticulum like cells and extensive keratin formation was observed. Peripheral cells were palisaded, cuboidal to tall columnar cells with nuclei showing reversal of polarity. The odontogenic epithelium was thrown into polypoid proliferations projecting into the stroma. A plexiform growth pattern predominated in most of the fields studied along with transverse sections of the papillae resembling follicles in the stroma. Our case was thus diagnosed as a ‘keratopapilliferous ameloblastoma’ taking into account all the features presented histologically.
The histologic differential diagnosis of PKA includes acanthomatous ameloblastoma, well differentiated Squamous Cell Carcinoma (SCC), ameloblastic carcinoma and primary intraosseous carcinoma.
Acantomatous ameloblastoma is charcterised by squamous metaplasia of the central areas of the ameloblastomatous follicles. It differs from PKA as it most commonly is seen in follicular variant of ameloblastoma and generally a layer of stellate reticulum like cells are present which separate the peripheral columnar cells from the central squamous cells [12]. Papillary projections have seldom been observed or reported as a feature in the acanthomatous variant.
The presence of keratin pearls in the areas of squamous metaplasia, acantholysis and peripheral palisading may depict a characteristic histologic picture of a well differentiated squamous cell carcinoma. But the absence of mitoses, varying degrees of nuclear and cellular pleomorphism, other dysplastic features and presence of reversal of polarity distinguishes PKA from squamous cell carcinoma [13].
Ameloblastic carcinomas are hypercellular lesions with sheets of mitotically active basaloid cells with hyperchromatic nuclei. The tall columnar peripheral cells show marked hyperplasia and the central squamoid or polygonal cells exhibit cellular and nuclear pleomorphism in addition to degenerative necrosis both in the epithelial and stromal component. Budding and anastomosing odontogenic epithelial processes are evident along with vascular and neural invasion; all of which differentiate ameloblastic carcinoma from PKA [14].
Primary Intraosseous Carcinomas (PIOC) histologically are similar to squamous cell carcinomas with islands of neoplastic squamous epithelium in a fibrous stroma with varying amounts of diffuse lymphocytic infiltration and extent of keratinization. PIOCs more commonly present as moderately differentiated SCCs without prominent keratin formation [14].
Incidence and recognition of various histopathologic variants of SMA is only of diagnostic importance as they have little bearing on the clinical behaviour of the tumour. But the same cannot be said for keratopapilliferous variant of SMA as very few cases (<7) have been reported worldwide. Moreover one of the cases documented by Collini developed a recurrence with histologic features which prompted the authors to rename the recurrence as an ameloblastic carcinoma [7].
More research regarding this unique variant including studies on immunohistochemistry, ultrastructure, molecular biology, or genetics are entailed for a better understanding of its features and for evaluation of its clinical behaviour.
Conclusion
Here, we report a case of papilliferous keratoameloblastoma which is a very rare variant of ameloblastoma. In the present case, clinical and radiographic findings and aspiration did not provide us any clue about the true nature of the lesion. Only on histopathology we could diagnose the lesion, thus affirming that histopathology stands out as a golden standard for pathologists. From what we know so far, the clinical behavior and treatment does not vary much from the conventional variant, except the one case which was later diagnosed as an ameloblastic carcinoma; we the pathologists should be aware of these unique variants. More case reports should elucidate the clinical features and biologic nature of these tumours in the future.
[1]. Bajpai M, Agarwal D, Bhalla A, Kumar M, Garg R, Kumar M, Multilocular unicystic ameloblastoma of mandibleCase Rep Dent 2013 :2013-83589. [Google Scholar]
[2]. Palaskar SJ, Pawar RB, Nagpal DD, Patil SS, Kathuriya PT, Keratoameloblastoma a rare entity: a case reportJCDR 2015 9(3):ZD05-07. [Google Scholar]
[3]. Whitt JC, Dunlap CL, Sheets JL, Thompson ML, Keratoameloblastoma: a tumour sui generis or a chimera?Oral Surg Oral Med Oral Pathol Oral RadiolEndod 2007 104:368-72. [Google Scholar]
[4]. Pindborg JJ, Pathology of the Dental Hard Tissues 1970 Philadelphia, USAWB Saunders:371-76. [Google Scholar]
[5]. Altini M, Slabbert HD, Johnston T, Papilliferous keratoameloblastomaJ Oral Pathol Med 1991 20(1):46-48. [Google Scholar]
[6]. Norval EJ, Thompson IO, vanWyk CW, An unusual variant of keratoameloblastomaJ Oral Pathol Med 1994 23(10):465-67. [Google Scholar]
[7]. Collini P, Zucchini N, Vessecchia G, Guzzo M’, Papilliferous keratoameloblastoma of mandible: a papillary ameloblastic carcinoma: report of a case with a 6-year follow-up and review of the literatureIntJ Surg Pathol 2002 10(2):149-55. [Google Scholar]
[8]. Mohanty N, Rastogi V, Misra SR, Mohanty S, Papilliferous keratoameloblastoma: an extremely rare case reportCase Rep Dent 2013 :706128 [Google Scholar]
[9]. Barnes L, Eveson JW, Reichert P, Sidransky D, World Health Organisation. Classification of TumoursPathology and Genetics of Head and Neck Tumours 2005 IARC PressLyon:284 [Google Scholar]
[10]. Adeyemi BF, Adisa AO, Fasola AO, Akang EE, Keratoameloblastoma of the mandibleJ Oral MaxillofacPathol 2010 14:77-79. [Google Scholar]
[11]. Takeda Y, Satoh M, Nakamura S, Ohya T, Keratoameloblastoma with unique histological architecture: an undescribed variation of ameloblastomaVirchows Arch 2001 439(4):593-96. [Google Scholar]
[12]. Kessler HP, Intraosseous ameloblastomaOral Maxillofacial Surg Clin N Am 2004 16:309-22. [Google Scholar]
[13]. Gnepp DR, Diagnostic Surgical Pathology of Head and Neck 2009 2nd editionPhiladelphiaWB Saunders:802-03. [Google Scholar]
[14]. Shafer WG, Hine MK, Levy BM, A Textbook of Oral Pathology 1993 4th editionPhiladelphiaWB Saunders:276 [Google Scholar]