Thyroid nodules occur in up to 50% of the normal adult population and most of them are not detectable by physical examination [1]. However, only 7% of the thyroid nodules are malignant and it is critical that they are accurately identified [2]. Palpable nodules occur only in 4% to 7% of the population. Small nodules of 2mm size can be detected by high-resolution ultrasonography (US) which can be missed by physical examination [3]. Most of these nodules are benign and on further evaluation, approximately 2% to 12% of them were found to represent malignancy [4]. The importance of ultrasound is to efficiently and effectively diagnose the minority of patients with thyroid malignancy [5]. High resolution ultrasound (US) is the most sensitive imaging test available for the examination of the thyroid gland. CT and MRI are more helpful for detecting local extension and extrathyroid lymphadenopathy. So, the imaging modality of choice for evaluating thyroid nodules is ultrasound.
Since US is more sensitive than physical examination, patients with palpable nodules are more commonly evaluated by US as a first step [6]. Ultrasound evaluation can evaluate the presenting nodule along with the rest of the thyroid to mark other non palpable nodules, if present [7]. US can also be used to guide percutaneous interventions [8]. US can help in preoperative planning and can demonstrate local invasion and lymph node involvement. There are a vast number of studies about the role of gray scale (B-mode) US and color Doppler US in the diagnosis of malignant thyroid nodules in the literature. Most of these studies say that color Doppler or a combination of color Doppler and B-mode US features were not as useful as the use of suspicious B-mode US features alone for predicting thyroid malignancy [9–18]. Few recent studies have reported that spectral Doppler is useful in diagnosing malignant nodules [5,10,19]. However, some authors state that vascular patterns or the Resistive Index (RI) values are not effective in differentiating between benign and malignant nodules [9,15–17,19]. In the present study, we seek to establish the specific gray scale and Doppler characteristics in differentiating malignant from benign thyroid nodules and to compare it with previous studies.
Materials and Methods
Study Population
The prospective case control study was conducted over a period of 2 years starting from March 2013 till April 2015 after approval from institutional ethics committee. The study sample included 214 thyroid nodules in 194 patients who had undergone ultrasound examination. USG guided targeted FNAC of 178 lesions was performed. Rest 16 nodules underwent surgery and histopathology details were obtained from surgical thyroidectomy specimen. Twenty cases were excluded from the study due to loss of follow-up/no histopathological confirmation. Thyroid nodules were broadly classified into benign and malignant category. One hundred fifty one thyroid nodules were benign and 43 were malignant. In the cases of multiple thyroid nodules, only one nodule was selected for each patient in US-FNA on the basis of the highest likelihood of malignancy, solidity and the largest diameter. We obtained written informed consent from all patients prior to each ultrasound guided FNAC. Patients with clinically palpable/non palpable thyroid nodules irrespective of size, who have not received any treatment which affect thyroid status (like antithyroid drugs). Patients with undetermined, inadequate, or suspicious malignant cytology from FNAC and patients who underwent FNAC before ultrasound were excluded from the study. Information regarding name, age, sex, inpatient number, associated risk factors like family history and history of irradiation, clinical symptoms of the patients and clinical diagnosis were obtained from all subjects by standard questionnaire and medical records. After collecting the above data, patients were subjected to both gray scale and Doppler imaging of the thyroid lesions after obtaining the informed consent. The equipments used were: GE Voluson expert with probe frequency 6-12 MHz / PHILIPS IU-22 with probe frequency 5-12 MHz / TOSHIBA Applio XG with probe frequency of 7.5 MHz. Both lobes of thyroid with isthmus were evaluated in succession for thyroid nodules, initially with gray scale and then color and spectral Doppler.
B-Mode Ultrasonography
Each nodule was evaluated using the gray scale ultrasound and was described according to the size, number, contents, echogenicity, margins, halo, shape, calcification, local infiltration and lymphnode enlargement. The nodule was described as solid, cystic or mixed (if the cystic component occupied less than 25% area, it was considered as solid; between 25 and 74% as mixed; and 75 and 100% as cystic) [5]. The echogenicity of the nodule was described as anechoic, hypoechoic, isoechoic, hyperechoic, or mixed when compared to the thyroid parenchyma. The shape of the nodule was described according to taller than wide (round/ oval). The borders of the nodule were described as smooth or irregular. The calcification in the nodule was classified as no calcification, microcalcifications (≤2 mm), peripheral or central calcification (> 2 mm) and comet tail artifacts. The peripheral halo was described as present, incomplete halo or absent. Other ancillary findings like lymphadenopathy present or absent and local infiltration present or absent were also evaluated.
Color and Spectral Doppler US
All nodules were examined by Doppler to describe the vascular patterns. Nodules were identified as nonvascular, peripheral, central, or mixed (both peripheral and central vascularity). Nodules with vascularity were identified as peripheral, central, or mixed vascular. Resistive index (RI) and Pulsatility Index (PI) values were calculated from the arteries of thyroid nodules by the software of the US equipment. RI and PI values were calculated from at least two arteries if possible from each nodule and if there was no second artery to measure at the central or peripheral region of a nodule, a single measurement was taken.
US-Guided Fine Needle Aspiration
Fine needle aspiration was performed under US guidance after ultrasound. All slides were interpreted by an experienced cyto-pathologist who was blinded to the US findings. The final pathological diagnosis (benign or malignant) for each nodule was made based on the US-guided FNAC or surgical pathological examination (if available).
Ultrasound criteria were then divided into major and minor criteria for the diagnosis of malignant nodules. The major criteria’s were hypoechoic nodule, solid nodule, incomplete halo, ill defined borders, microcalcifications, lymphnode enlargement with loss of fatty hilum, local infiltration, intranodular vascularity and high RI and PI (>0.73 and 1.3). The minor criteria were taller than wide nodule, no halo, mixed echogenicity, macrocalcifications and mixed vascularity. TIRADS Classification was not applied since it does not include spectral parameters of Doppler ultrasound. Finally, each set of images were independently reviewed by a radiologist without knowledge of the clinical or biopsy results.
Statistical Analysis
Statistical analysis was performed using SPSS software. Cyto-pathological results of each nodule were compared with ultrasound findings and FNAC/Biopsy results. Student’s t-test was used for comparison between groups. Pearson Chi-Square test was used to determine the association among physical examinations, findings of US and Cyto-pathological examinations. Receiver Operating Characteristic (ROC) curve analysis was performed to establish cutoff, sensitivity and specificity values and mean RI-PI values. Doppler indexes of non-vascular nodules could not be measured; therefore, they were not counted in the statistical analysis concerning RI-PI values. Statistical significance was set at p <0.05.
Results
Mean patient age was 41.5±9.65 and 49.3±10.4 years in patients with benign and malignant nodules, respectively. Overall nodule diameter ranged from 4-67 mm in the greatest dimension (mean: 27.04 mm; standard deviation (SD): 11.5 mm) [Table/Fig-1]. Cyto-pathological results were similar in both sexes and different age groups (p > 0.05).
Demographic details (age and sex) of the thyroid nodules in current study.
| Cytology | Number ofnodules | Sex ratio(Female / Male) | Mean age ± SD |
|---|
| Benign | 151 | 118/33 | 41.5 ± 9.65 |
| Malignant | 43 | 29/14 | 49.3 ± 10.4 |
| Total | 194 | 147/47 | 45.78 ±9.9 |
B-Mode US Evaluation
A solitary nodule was found in 90 and multi-nodular goiter was found in 104 of 194 cases. No correlation was found between the cyto-pathological results and the number of nodules (p > 0.05). Mean nodule diameter was 25.7 ± 6.5mm in benign nodules and 33.6 ± 4.8 mm in malignant nodules. Significant relationship was observed between malignancy and irregular margins, microcalcifications, hypoechogenicity, taller than wide, lymphnode enlargement with loss of fatty hilum and local infiltration (p <0.05) [Table/Fig-2]. Most of the thyroid nodules had solid contents which were seen in both benign and malignant nodules with insignificant p-value. Majority of the cystic thyroid nodules were benign in nature. Most of the isoechoic and hyperechoic nodules were seen in benign thyroid nodules. Hypoechoic nodules were most commonly seen in malignant nodules with a six fold increase for malignancy than iso-hyperechoic nodules. Ill defined margins were commonly seen in malignant thyroid nodules which was a specific criterion for differentiating malignant and benign nodules.
Comparison of B mode ultrasonographic findings of benign and malignant nodules.
| Nodule features | Benign (151) | Malignant (43) | p-value |
|---|
| Solitary nodule | 90(46.39%) | 104(53.61%) | 0.0766 |
| Mean diameter | 25.7±6.5 | 33.6±4.8 | 0.0455 |
| Solid nodule | 93(67%) | 31(33%) | 0.172 |
| Hypoechogenicity | 31(45.58%) | 37(54.42%) | 0.0001 |
| Incomplete halo | 6(31.68%) | 13(68.42%) | 0.001 |
| Irregular borders | 26(49.06%) | 27(50.94%) | 0.012 |
| Taller than wide | 61(61%) | 39(39%) | 0.003 |
| Microcalcifications | 0 | 12(100%) | <0.05 |
| Lymphadenopathy with loss of fatty hilum | 0 | 17(100%) | <0.05 |
| Local invasion | 0 | 1(100%) | <0.05 |
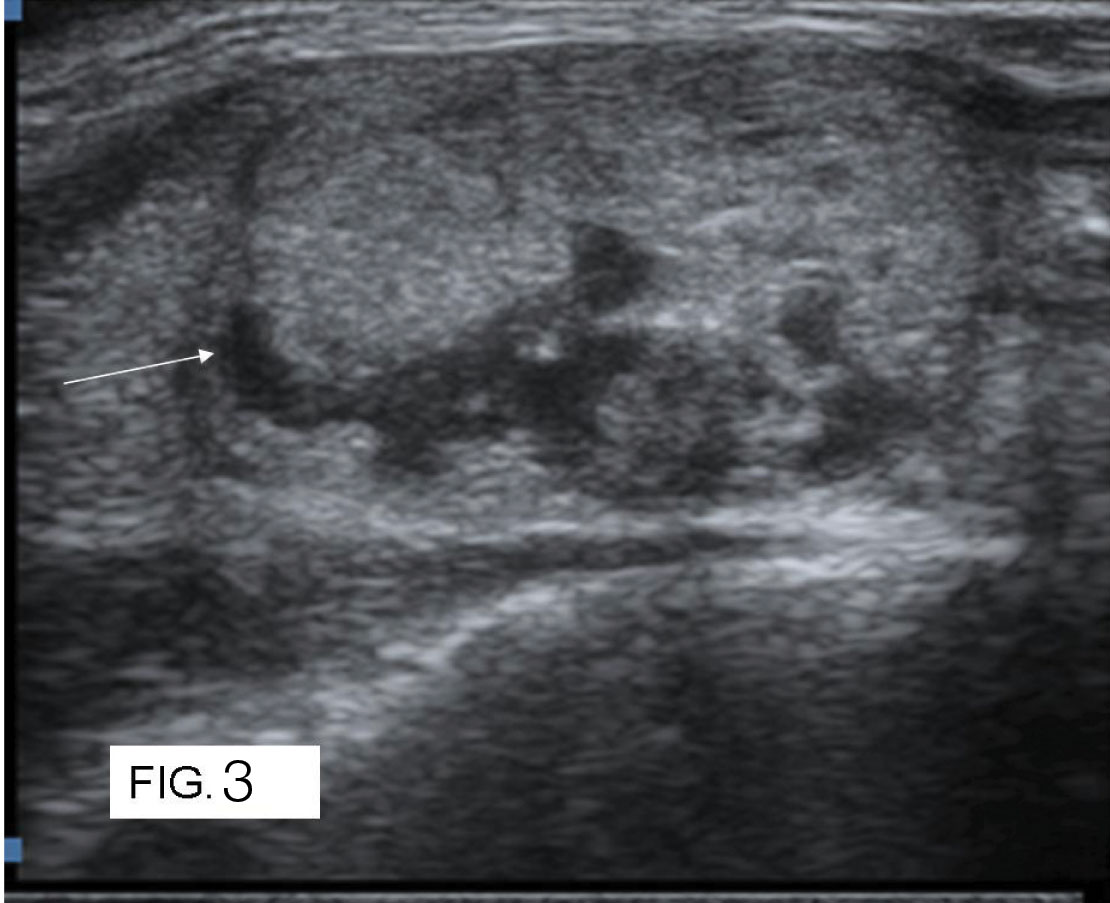
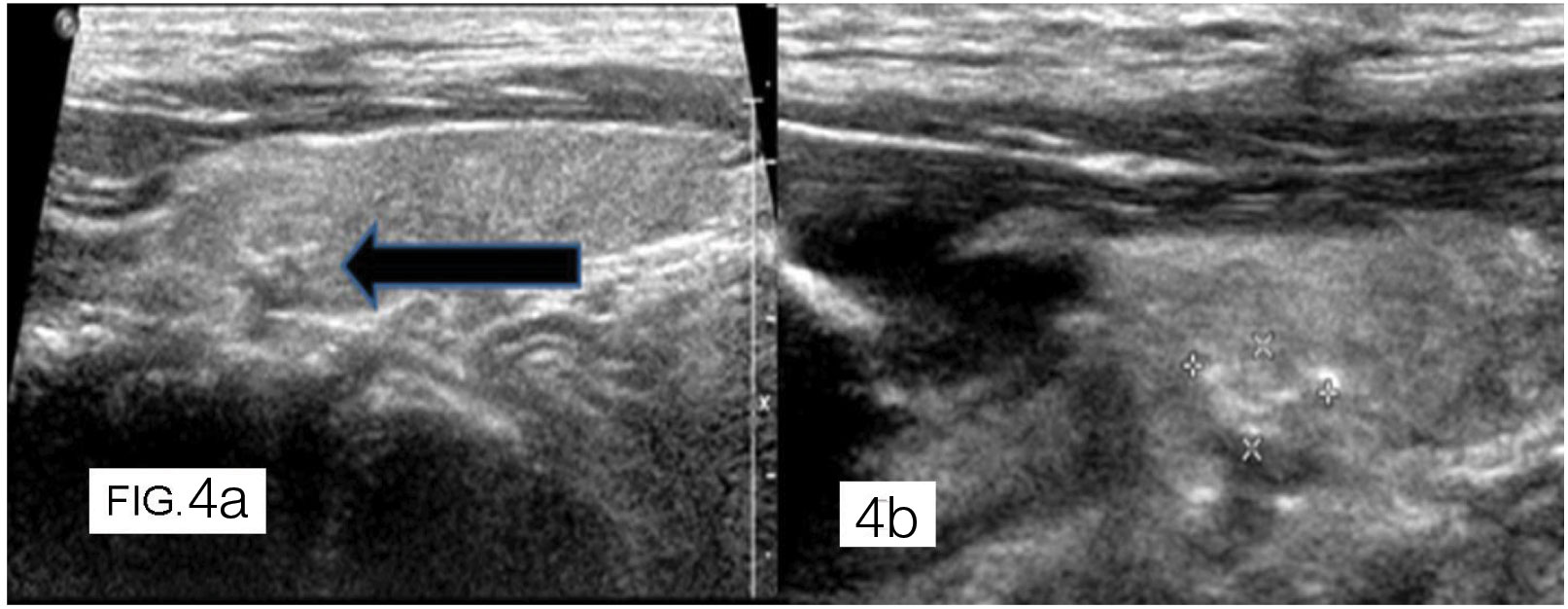
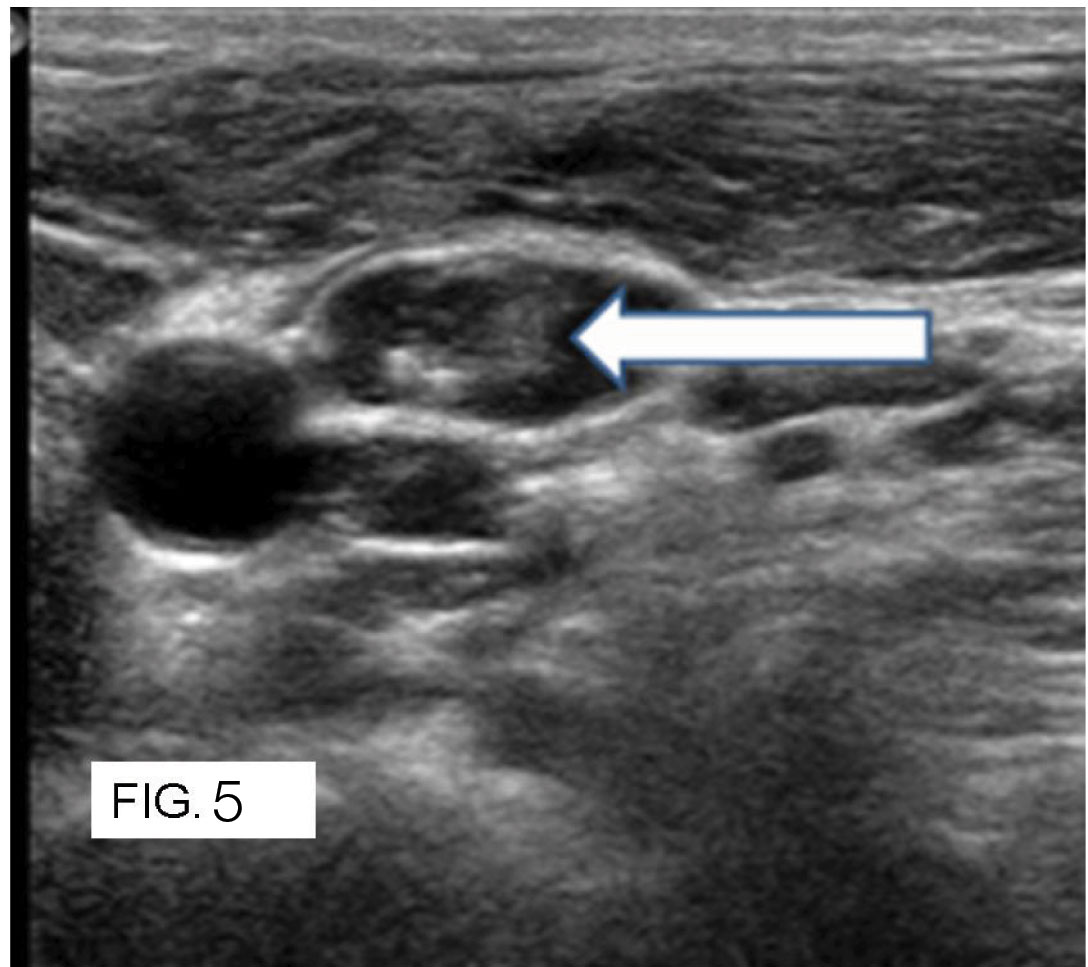
Absence of halo was seen in both malignant and benign thyroid nodules with insignificant p-value. Presence of halo was seen in majority of the benign nodules which showed a significant p-value in differentiating benign malignant nodules. Thick irregular halo was one of the specific features of malignant nodules which were not mentioned elaborately on literature [Table/Fig-3]. Significant numbers of the malignant nodules were taller than wide due to increase in the nutrient supply to tumour tissue which was a significant criterion in differentiating malignant from benign nodules. All cases of microcalcifications were seen in papillary carcinoma with 100% specificity [Table/Fig-4a,b]. Barring few cases of papillary and medullary carcinoma, majority of the macrocalcifications were seen in benign thyroid nodules (Adenomatous nodules) and showed insignificant p-value. Local invasion was a very specific ancillary criterion of malignant thyroid lesions in differentiating benign and malignant thyroid nodules, but the incidence was very less. Lymphadenopathy with loss of fatty hilum was seen only in malignant thyroid nodules majority of them are seen in papillary carcinoma [Table/Fig-5].
A 40-year-old female patient with follicular carcinoma: Grey scale ultrasound image shows ill defined isoechoic nodule with thick irregular halo (white arrow) in the right lobe of the thyroid gland.
A 43-year-old male patient with papillary carcinoma. Grey scale ultrasound image shows multiple ill defined foci of microcalcifications (thick black arrow and asterisks) in left lobe of thyroid gland.
A 48-year-old female patient with papillary carcinoma. Grey scale ultrasound image shows well defined hypoechoic enlarged lymphnode with loss of fatty hilum and foci of microcalcifications (thick white arrow).
Based upon the gray scale characteristics, the nodules were classified into benign and malignant. Results were tabulated below [Table/Fig-6].
Comparison of the results of the diagnosed nodules by the gray scale ultrasonography with histopathology confirmed nodules (HX).
| Gray Scale Characteristics | HX confirmedMalignant | HX confirmedBenign | Total |
|---|
| Malignant | 36 (TP) | 20 (FP) | 56 |
| Benign | 7 (FN) | 131 (TN) | 138 |
| Total | 43 | 151 | 194 |
TP-True positive, FP- False positive, FN-False negative, TN-True negative, HX- histopathology confirmed.
Color Doppler
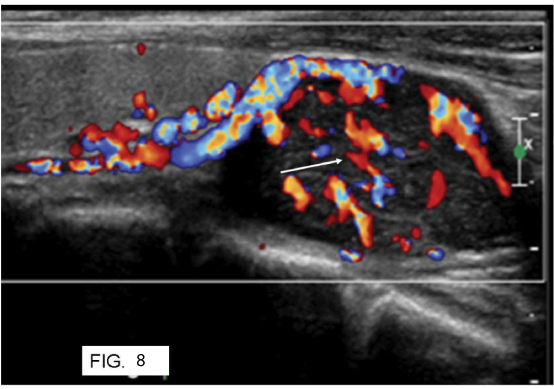
Of the 194 nodules, 121 nodules showed perinodular flow, 37 nodules showed intranodular flow and 33 cases showed both intranodular and perinodular flow [Table/Fig-7]. 3 nodules showed no vascularity. Of the 33 nodules with both intranodular and perinodular flow, 28 nodules were benign and 5 nodules were malignant. Of the 36 malignant nodules with intranodular vascularity, 19 nodules were papillary carcinoma, 9 nodules were follicular carcinoma, 7 nodules were medullary carcinoma and 1 nodule was metastases. Only one benign nodule showed intranodular vascularity. Intranodular vascularity was one of the specific finding for malignant nodules in differentiating benign and malignant thyroid nodules in this study [Table/Fig-8].
Distribution of different vascular patterns among benign and malignant thyroid nodules.
| Flow type | Benign | Malignant | Total |
|---|
| Central | 1(0.66%) | 36 (83.72%) | 37 |
| Peripheral | 119 (78.83%) | 2 (4.65%) | 121 |
| Mixed | 28 (18.5%) | 5 (11.62%) | 33 |
| No flow | 3 (1.9%) | 0 | 3 |
| Total | 151 | 43 | 194 |
A 58-year-old female patient with medullary carcinoma. Color Doppler ultrasound image shows a well defined hypoechoic nodule with internal vascularity.
Based upon the color Doppler characteristics, the nodules were classified into benign and malignant. Results were tabulated in [Table/Fig-9].
Comparison of the results of the diagnosed nodules by the color Doppler ultrasonography with histopathology confirmed nodules.
| Color Doppler | Malignant (HX) | Benign (HX) | Total |
|---|
| Malignant | 41 (TP) | 29 (FP) | 70 |
| Benign | 2 (FN) | 119(TN) | 121 |
| Total | 43 | 148 | 191 |
Spectral Doppler
In spectral Doppler only RI and PI were taken into account for differentiating the benign from malignant thyroid nodules. The PSV and EDV were not taken into account as they have no significance. So, RI values greater than 0.73 were considered malignant and less than 0.73 were considered benign. PI values greater than 1.3 were considered malignant and less than 1.3 was considered benign [Table/Fig-10]. Among the RI values > 0.73, about 33 cases accounting to 76.74% were seen in malignant thyroid nodules. The rest 22 cases accounting to 14.86% belong to the benign thyroid nodules. In the present study, RI values less than 0.73, were seen in 23.26% of the malignant nodules and 85.14 % of the benign thyroid nodules. In the present study, PI values > 1.3 were seen in 26 malignant cases (43%). The rest 34 cases were seen in benign nodules (57%). In current study, PI values less than 1.3 were seen in 17 malignant cases (13%). The rest 114 cases were seen in benign nodules (87%). Based upon the spectral Doppler characteristics, the nodules were classified into benign and malignant. Results were tabulated below [Table/Fig-11,12].
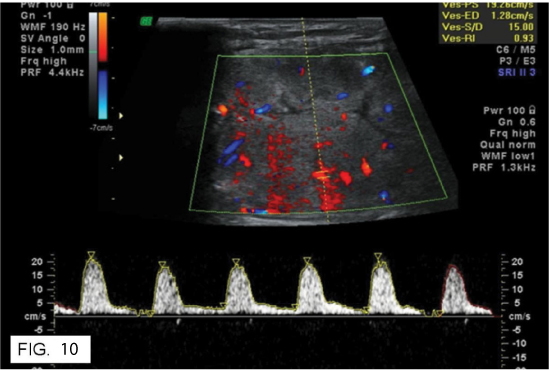
A 61-year-old female patient with follicular carcinoma. Spectral Doppler showing high RI and PI values and report was given as malignant nodule.
Frequency of benign and malignant nodules detected by RI values.
| RI | Malignant | Benign |
|---|
| > 0.73 | 33 (76.74%) | 22 (14.86%) |
| < 0.73 | 10 (23.26%) | 126 (85.14%) |
| Total | 43 | 148 |
Frequency of benign and malignant nodules diagnosed by PI values.
| PI | Malignant | Benign |
|---|
| > 1.3 | 26 (43%) | 34(57%) |
| < 1.3 | 17 (13%) | 114 (87%) |
| Total | 43 | 148 |
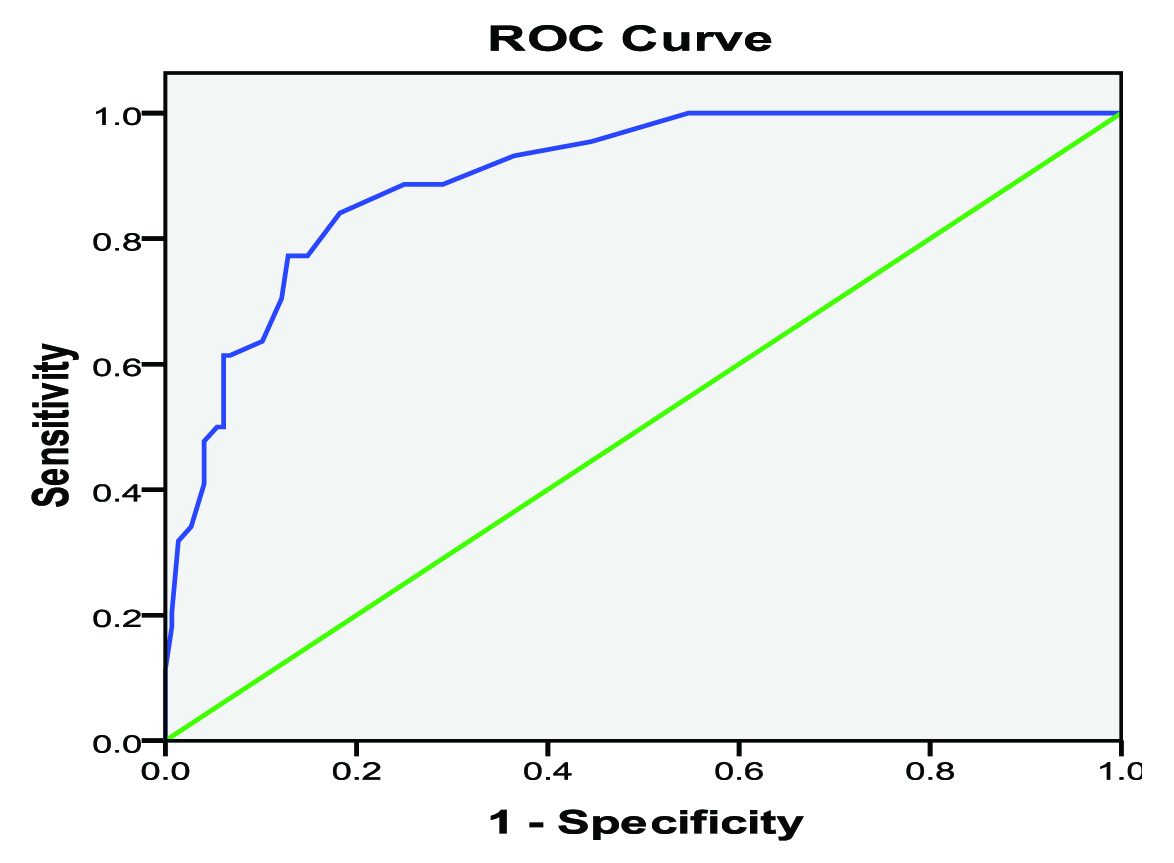
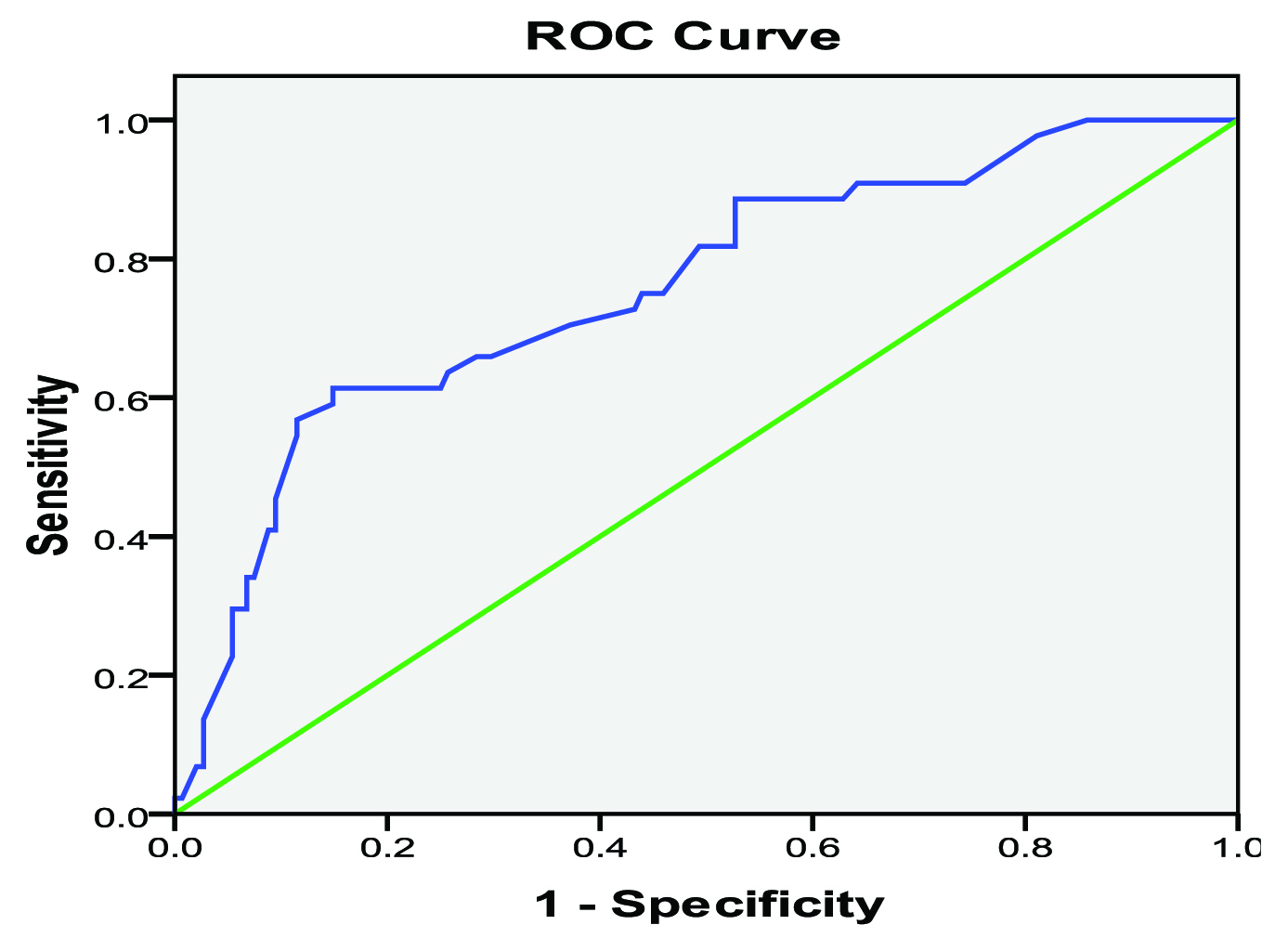
Receiver operating characteristics (ROC) curves were derived for Doppler parameters to assess sensitivity and specificity for various RI and PI cutoff values [Table/Fig-13,14].
Receiver operating characteristics curve shows relationship between sensitivity and specificity of RI for each cut-off value.
Receiver operating characteristics curve shows relationship between sensitivity and specificity of PI for each cut-off value.
Fine Needle Aspiration Cytology and Surgical Thyroidectomy Results
Eventually, 151 and 43 nodules were diagnosed as benign and malignant, respectively. Out of 151 confirmed cases of benign nodules, 66 cases were degenerated goitre, 45 cases were adenomatous goitre, 23 cases were colloid goitre and 18 were lymphocytic thyroiditis. Out of 43 confirmed cases of malignant nodules, 23 were papillary carcinoma, 12 were follicular carcinoma, 8 were medullary carcinoma, 1 was anaplastic carcinoma and metastases.
Discussion
A thyroid nodule is a manifestation of a gamut of thyroid diseases and it is important to recognize reliable criteria for malignancy in a thyroid nodule when using imaging methods [20]. The exact nature of thyroid nodule is ultimately established by histopathological examination. According to the literature, FNAC cannot be done on all incidentally detected thyroid nodules as it is expensive and not practical. It is suggested, that for nodules less than 10mm, only those with a high-risk of malignancy or suspicious US features must be biopsied. Present study proposed that age and sex incidence is not an important criteria in differentiating benign and malignant thyroid nodules which is in consensus with other studies [2,5,21]. Our study concluded that nodule number and size is not predictive of malignancy because the likelihood of cancer in a thyroid nodule has been the same regardless of the size of the nodule measured during ultrasonography which substantiates other studies [2,5,21]. The relationship between solid nodule and malignancy was not statistically significant in our study which is in accordance with the findings of Algin et al., [5]. While the presence of cystic components in a thyroid lesion was found to be a significant criterion (p<0.05) for benign nodules in our study, which was not in agreement with the findings of lannuccilli et al., [9]. Current study also showed that the risk of malignancy in hypoechoic nodules is about 6 times higher than the iso-hyperechoic nodules [5,21]. Our present study states that hypoechogenicity, irregular margins, microcalcifications and taller than wide are important features in determining the malignancy risk which is in consensus with other studies [2,5,9,20–23]. The present study showed significant p-value for presence of halo in benign nodules which substantiates the findings of other studies.
Current study and other study done by Algin et al., showed the presence of halo alone for detection of malignant thyroid nodules was insignificant (p>0.05) [5]. In our study, a new criteria which is thick irregular halo was seen in 13 malignant nodules (69 %) and 6 benign nodules (31%). This characteristic was not evaluated in other studies. In current study, the p-value for thick incomplete halo was significant (p<0.05). This can be attributed to irregular growth and infiltration of the fibrous capsule by the malignant thyroid nodule leading this irregular halo. In present study microcalcifications were seen in 12 nodules of papillary carcinoma. However, none of the benign nodules were seen to have microcalcifications. The positive predictive and negative predictive value of microcalcifications alone for detection of malignant thyroid nodules was 100 % and 82.1%. The p-value for microcalcifications was found to be 0.001 was significant (p<0.05) [5,20,22]. In present study, comet tail artifacts were seen in seen in 23 nodules of which all are benign (having specificity of 100%). Hence, this is a specific criterion for benign thyroid nodules. This finding of present study was comparable with the findings of the Wong and Ahuja, who concluded that comet tail artifacts are a sign of benignity [24]. Current study states that lymphnode enlargement with loss of fatty hilum and local infiltration are important features in determining the malignancy risk which was a high specificity and positive predictive value of 100%.
The sensitivity of Doppler US investigation is affected by settings of a wall filter, depth of the nodule and pulse repetition frequency (PRF), by variations of tissue attenuation, patient movement and lack of cooperation, swallowing or breathing motions. Pulsations of adjacent arterial structures may also affect Doppler US investigation. But recent studies suggested that parameters identified by color and spectral Doppler US may be able to differentiate between malignant and benign nodules [5,10,19]. Although few other studies reported contradictory results thyroid nodules are classified as non-vascular, peripheral vascular, central vascular, and mixed vascular according to the Doppler examination findings [9,15–17,19]. Increased central vascularity is generally accepted as a supporting feature for diagnosis of malignancy in the literature [5,10,19]. In our study, we found significant relationship between central vascularity and malignancy. Studies done by Moon et al., [17], Argalia et al., Tamsel et al., and Rosario et al., did not find any relationship between intratumoural vascularity and malignancy, which is not in consistent with the results of our study [25–27]. However, study done by Mohammadi et al., says that intranodular vascularity is an important predictor of malignancy [28].
Thyroid nodules assessment by RI and PI values is not affected from course of artery, angle of insonation, or nodule size. However, blood velocity measurements may be altered by Doppler parameters chosen by the examiner. Generally blood velocity parameters in the diagnosis of thyroid cancer are not considered useful. Hence, we analyzed only RI and PI values instead of velocity measurement in our study. The present study found that malignant nodules have significantly higher RI and PI values compared to benign nodules. Other studies by Argalia et al., De Nicola et al., and Ivanac et al., demonstrated similar results for RI values like our study [25,29,30] The high central and peripheral RI-PI values noted may be due to stenosis and/or occlusion of arteries due to excess cellular proliferation in malignant nodules. We believe the influence of these vascular indexes should be tested with further comprehensive studies with a larger patient cohort. Comparison of gray scale, color, spectral Doppler and the combined sonography in detecting sensitivity and specificity, negative/positive predictive values, cut-off values of RI-PI indexes for diagnosis of malignancy are summarized in [Table/Fig-15].
Accuracy of gray scale, color, spectral Doppler and the combined sonography.
| Modality | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|
| Gray | 83.72% | 86.75% | 64.28% | 94.9% | 86.08% |
| COL | 95.34% | 80.4% | 58.57% | 98.34 % | 83.76 % |
| SPE | 76.78% | 81.7% | 55% | 92.36% | 80.62% |
| GR+DOP | 94.59 % | 88.9% | 68.62% | 98.47% | 90.10 % |
GR: Gray scale, COL: color Doppler, SPE: spectral Doppler, DOP: Doppler
Limitation
There are few potential limitations of this study. The main limitation is few overlapping features were seen in both benign and malignant thyroid nodules. In patients with multinodular goitre, only the suspicious and dominant nodules were further evaluated by FNAC. Rest of the nodules was not evaluated. Small nodules less than 10 mm could not be assessed properly on color Doppler. Small sample size for individual malignant thyroid lesions. Sonoelastography was not used in this study which would have increased the sensitivity and specificity when combined with grey scale, color and spectral Doppler.
Conclusion
Ultrasonography has resulted in uncovering a spectrum of clinically unapparent thyroid nodules, the overwhelming majority of which are benign. Using morphological pattern recognition features, ultrasound is valuable for identifying malignant or potentially malignant thyroid nodules. Recognition of specific morphologic patterns helped in differentiating malignant from benign features. The specific malignant features include microcalcifications, hypoechogenicity, taller than wide, irregular thick halo, lymphadenopathy and local extra thyroidal invasion. Other features such as the absence of a halo, macrocalcifications and solid composition are less specific but may be useful ancillary signs. Intranodular vascularity and high RI indices were few of the specific signs for malignant thyroid nodules in our study. Since Gray scale and Doppler have their own strengths and weaknesses, they were complementary rather than competitive modalities in diagnosing benign from malignant thyroid nodules. We believe combinations of grey scale, color and spectral Doppler would improve the detection of malignancy in thyroid nodules and can guide us to recommend for FNAC.
GR: Gray scale, COL: color Doppler, SPE: spectral Doppler, DOP: Doppler