Dengue is one of the major causes of thrombocytopaenia. It can lead to severe thrombocytopaenia resulting in massive internal haemorrhage. Thus, to prevent the lethal outcome, the dengue patients are often hospitalized and serial platelet counts are monitored. When the platelet count is severely low, prophylactic platelet transfusions are given to circumvent haemorrhagic manifestations [1–3].
A platelet unit refers to one aphaeresis platelet unit or a pool of 4 to 6 whole blood-derived platelet concentrates, containing 3 to 4 × 1011 platelets [1]. In case of adults, six units of random donor pooled platelets can be transfused in 20-30 minutes. The post-transfusion platelet count reaches a peak after 10 minutes to one hour and begins to wane after 72 hours [3].
Single donor platelet units are expensive and are available in only few advanced health care centers as sophisticated apheresis equipment is needed to retrieve the platelets from the donors. Random donor platelets are obtained from whole blood after component separation and hence are relatively inexpensive. They are accessible even to rural health care centers which are not equipped with apheresis units. Thus, required number of platelet units can be issued at minimum costs and multiple platelet transfusions can be done if necessary. However, the disadvantage of random donor platelet unit is that they are issued without considering the blood group of the recipient as they have a shelf life of only 5 days and their resources are scarce. The pooled platelet units can be issued randomly without regard to the patient’s blood group except in case of women of child bearing age who received Rhesus D compatible platelet units to prevent RhD alloimmunization [4,5].
‘ABO-compatible’ refers to the fact that the donor has no A or B antigens incompatible with the recipient’s A or B antibodies. Platelets express ABH antigens, which can minimize platelet transfusion recovery and survival in ABH-incompatible recipients. ABO and Human Leukocyte Antigen (HLA) compatible platelets result in a greater platelet count increment in the recipient, and therefore are used in cases of platelet refractoriness [4,5].
Many studies have been conducted on effectiveness of platelet transfusion on haematology-oncology patients with thrombocytopaenia, utilizing single donor apheresis units [6–9]. However, very few studies have documented the effects of pooled platelet transfusion on platelet count, especially in dengue-related thrombocytopaenia patients. In this study, we evaluated the platelet count changes in dengue patients after random donor pooled platelet transfusion as these platelet concentrates are widely available, commonly used in developing countries and are useful in the absence of haematological malignancies. We compared the changes in platelet counts in patients who received transfusion from same blood group with the patients who received the transfusion from other blood groups.
The present study was conducted with the aim to compare the platelet count changes in the concordant and discordant groups that had received ABO compatible and ABO incompatible platelet units respectively.
Materials and Methods
This was a retrospective study performed using platelet counts recorded before and after random donor pooled platelet transfusions that took place during the period of 2.4 years, from May 2013 to September 2015, at Sapthagiri Institute of medical Sciences and Research Centre, Bangalore.
The platelet counts recorded before and after platelet transfusion in adults of the age 12 years and above admitted for dengue fever or dengue haemorrhagic fever with platelet counts less than 10,000/μl and those with higher platelet counts associated with bleeding manifestations was the inclusion criteria.
The outcome of platelet transfusion in patients with thrombocytopaenia due to other causes, children aged less than twelve years, pregnant women, patients with splenomegaly, patients on ayurvedic or homeopathy medications along with platelet transfusion, recipients of packed red cells on the same day of platelet transfusion and recipients of multiple platelet transfusions within 24 hours were excluded.
Patients with thrombocytopaenia were transfused with platelets procured from whole blood using component separation. Each patient received four units of platelets in one sitting, each unit containing 63ml platelets suspended in plasma.
The Directorate of Health Services (DHS) guideline for the prophylactic platelet transfusion was used in this study. Dengue patients with platelet counts <10000/μL with or without bleeding manifestations received prophylactic platelet transfusions [10].
All the platelet units were screened for transfusion transmitted diseases. The pooled platelet units were issued randomly without regard to the patient’s blood group except in case of women of child bearing age who received Rhesus D compatible platelet units to prevent RhD alloimmunization [4]. However, blood group of the recipients and the ABO group of the issued platelet units were documented in the registers of the blood bank.
The single donor platelets contain 4-8 times more plasma than random donor platelets and hence minor cross match has to be performed before transfusion [4]. However, they were not used in this study.
Among the recipients of multiple platelet transfusions the outcome of only the first transfusion was taken into consideration. The results of the subsequent transfusions were not included as most of these patients had received packed cells along with platelet concentrates and also due to non-availability of follow up at 24 hours in remaining cases. Also, those who received more than one transfusion consisting of 4units within 24hours were excluded from the study as it would alter the platelet count changes at 4 hours and 24 hours.
Demographic data, history, and clinical examination findings of all patients were recorded, and blood samples were drawn to measure the platelet counts. The platelet counts were noted at admission and while being monitored in hospital. The clinical features at admission and other medical treatment received were noted. The height, weight, viral serology, and complete blood counts were noted along with findings of ultrasonography of abdomen.
Blood samples were collected in Ethylene Diamine Tetra-Acetic Acid (EDTA) anticoagulated vials and platelet counts were documented. Platelet counts were measured twice daily for all patients with dengue fevers and also before, at 4 hours and at 24 hours after platelet transfusions. Corrected Count Increments (CCI) was calculated using formula:
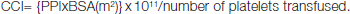
{PPI = post transfusion platelet increment (post transfusion platelet count minus pre transfusion platelet count); BSA= body surface area in square meters, formula used to calculate BSA - Mosteller formula}. PPI at 24h= platelet count at 24h-platelet count at baseline [3].
The outcome of a total of 203 platelet transfusions in 203 patients was analyzed in this study. These patients were classified into responders and non-responders depending upon the changes in post transfusion platelet counts. Patients with PPI ≥10000/μL and/ or CCI ≥5000/μL following transfusion were classified as responders; the rest were classified as non responders [3].
Platelet count changes in patients receiving ABO compatible platelets were compared with those receiving ABO incompatible platelets. Paired t-test was used for statistical analysis of these two cohorts.
Results
In this study, the changes in platelet counts following random donor pooled platelet transfusion were evaluated. The platelet count changes in patients who received transfusion from same blood group and the patients who received the transfusion from other blood groups were compared.
Out of the 203 patients enrolled in this study, 159 (78.32%) patients experienced improvement of platelet counts. These cases were called as responders. Among these, 99 (62.26%) patients had received ABO identical platelet units and 60 (37.73%) patients had received ABO compatible platelet units.
Among the remaining 44 (21.6%) patients, 10 (4.9%) cases showed no change of platelet counts. 20 (9.8%) patients showed minimal increments i.e., PPI <10000/μl and 14 (6.8%) patients experienced decrease in the platelet counts. These 44 cases were classified as non responders.
Among the 20 non-responders who showed minimal increments in post transfusion platelet counts (PPI<10,000/μL), 13 (65%) patients received one ABO identical platelet unit and 7 (35%) received one ABO compatible platelet unit along with three incompatible units.
Among the 10 patients whose platelet counts remained constant after transfusion 6 (30%) patients had received ABO identical platelets, and 4(40%) patients, ABO compatible platelets.
All the 14 (6.8%) patients who experienced a decrease in platelet counts following transfusions had received ABO incompatible platelet units.
Thus in this study all the responders had received either ABO identical or ABO compatible platelet units. Those patients who showed minimal increments in platelet counts also had received at least one ABO compatible or identical platelet units.
Lack of increments in platelet counts following ABO identical or ABO compatible platelet transfusion was observed in 13.63% of non responders, which was probably due to virus induced destruction of platelets.
Post-transfusion platelet increments and corrected count increments were higher in responders as compared to non-responders.
The median Post transfusion Platelet Count Increment (PPI) and Corrected Count Increment (CCI) at 4hour post transfusion were 25,000/μL {inter quartile range (IQR) 5,000-80,000/μL} and 18,000/μL (IQR 8,000/μL- 47,500/μL) respectively among the responders. Median PPI and CCI at 24 hours were 45,000/μL and 28,863/μL among the responders. The median CCI at 4 hour post transfusion among the non-responders was 850/μL and at 24hours was 1,425/μL. At 24 hours responders showed significantly higher PPI as compared to non responders [Table/Fig-1]. Data are described in terms of median and Inter Quartile Range (IQR) as data is skewed.
Comparison between responders and non-responders.
| Responders Median (IQR)μ/L | Non-Responders Median (IQR)μ/L | p-value |
|---|
| Platelet count at baseline | 15,000 (12,000-20,000) | 16,000 (10,500-20,000) | 0.903 |
| Platelet count at 4hour | 41,000 (30,000-52,000) | 15,000 (12,000-18,000) | < 0.001 |
| Platelet count at 24hour | 65,000 (50,000-1,00,000) | 12,000 (9,000-18,000) | < 0.001 |
| CCI at 4hour | 18,000 (13,500-23,869) | 850 (475- 900) | < 0.001 |
| CCI at 24hour | 28,863 (21,250-43,125) | 1,425 (850-1,912) | < 0.001 |
| PPI at 4hour | 25,000 (15,000-35,000) | 0 (-3,000)-(-2,000) | < 0.001 |
| PPI at 24hour | 45,000 (35,000-80,000) | 0 (-9,000)- (3,000) | < 0.001 |
| Spontaneous platelet recovery | 20,000 (10,000-40,000) | 0 (-3,000)- (2,000) | < 0.001 |
| Comparison of Platelet count at baseline to Platelet count at 4hour | < 0.001 | 0.221 | ---- |
| Comparison of CCI at 4hour To CCI at 24hour | < 0.001 | < 0.001 | ---- |
There were no reported cases of haemolytic transfusion reactions in the study population. Patients who received incompatible platelet transfusions needed multiple platelet transfusions to maintain stable platelet count of > 10,000/μL or to prevent bleeding episodes.
Discussion
Although according to some of the studies, there is no role for prophylactic platelet transfusion therapy in dengue patients, many clinicians transfuse platelets to correct thrombocytopaenia during the critical phase of recovery from dengue infections to avoid haemorrhagic complications [1–3]. Dengue virus-induced bone marrow suppression and an immune mechanism of thrombocytopaenia results in severe thrombocytopaenia in patients with Dengue Hemorrhagic Fever (DHF) [3].
Single-donor platelets retrieved from apheresis and random donor pooled platelet concentrates have a shelf life of about 5 days, when stored at 22+/-2°C with similar efficacies in raising the post transfusion platelet counts [1].
In this study, majority of the responders received ABO identical random donor pooled platelets and the rest received ABO compatible pooled platelets. Among the non-responders those who received at-least one ABO compatible platelet unit showed minimal increments in post-transfusion platelet counts. The patients who received ABO incompatible platelets experienced decrease in post transfusion platelet counts.
Platelets (PLTs) are often transfused to adults without regard for ABO compatibility in order to overcome the shortage, as they have only 4-7 days of shelf life. The neutralization of the donors’ anti-A or anti-B antibodies by the recipients soluble or endothelial based A and B antigen(s) and or the dilution of the donor anti-A or anti-B antibodies in the recipient’s plasma volume have been thought to prevent haemolysis despite the presence of ‘high titer’ anti-A and anti-B antibodies in some of the donor units [5]. The advantages of ABO identical PLTs are not well documented in the literature. Administration of ABO-incompatible platelets is an acceptable transfusion practice [5].
Cooling LLW et al., (2008) demonstrated that including a single bag of non-group O whole blood-derived PLT unit in a pool of 3–4 group O PLTs decreased or neutralized, the anti-A and anti-B antibodies in the pool [11]. When the ABH antigens on the donor PLTs are coated with recipient anti-A and/or anti-B antibody, they are filtered off from the blood [5].
In this study, the median post transfusion Platelet Count Increment (PPI) and Corrected Count Increment (CCI) at 4hours and 24hours post transfusion were significantly higher among the responders than the non responders. The other studies that evaluated the post transfusion platelet count changes following platelet transfusions administered for thrombocytopaenia of various causes are tabulated in [Table/Fig-2].
Comparison between present study and various other studies that evaluated post transfusion platelet count changes following ABO compatible and incompatible platelet transfusions.
| Study | Study subjects | Type of platelet usedin the study group | CCI /μL |
|---|
| Compatible transfusion | Incompatible transfusion |
|---|
| Present study | Dengue patientsn=203 | ABO identical and compatible Random donor pooled platelets | Median CCI-28,863 (IQR,21250- 43125) | Median CCI-1,425 (IQR,850- 1912) |
| Pavenski K.,2010 [6] | Non oncology patientsn=1030 | ABO compatible single donor platelets | Median CCI- 18,600 (IQR, 10,200-28,400) | Median CCI -15,200 (IQR, 4,700-25,700) |
| Salama OS et al.,2014 [7] | Patients refractory to random donor plateletsn=40 | Cross match compatible platelets[SDP&RDP] | 23,280±15,870(Mean ±SD) | 11,980±7,510(Mean ±SD) |
| Elhence P et al.,2014 [8] | Oncology patientsn=31 | Cross matched single donor platelets | 9,250±026.6(Mean ± SD) | 6,757.94±2,656.5 (Mean ± SD) |
| Heal JM et al.,1987 [9] | Oncology and other haematological diseasesn=51 | Cross matched single donor platelets | 10000/μL | 5900/μL |
In a study conducted by Kulkarni N, 64 dengue patients with platelet counts less than 20,000/μL received multiple (10-12) units of random donor platelets. They showed an increment of > 50,000/μ L, however they have not measured it in terms of CCI or PPI [12].
In patients receiving bone marrow transplant, ABO-major mismatched PLT transfusions can lead to lower than expected post-transfusion PLT increments.
The other factors such as transfusion of packed cells on the same day of the PLT transfusion, the presence of recipient HLA antibodies, administration of corticosteroids, and large body surface area can lead to lack of increments in platelet counts [13].
Several studies have documented that the recipients of ABO-identical PLT transfusions had higher absolute post-transfusion PLT increments compared to recipients of ABO-major mismatched PLT units [6–9]. This was observed in this study also.
Another randomised control study carried out on 1272 haematology oncology patients evaluated 4hour post transfusion platelet increments in 3993 transfusions and 24 hour post transfusion increments after 2309 transfusions. They recorded similar outcomes [14]. In the present study only serologically proven dengue cases were enrolled and higher post transfusion platelet count increments were noted in recipients ABO compatible pooled platelet transfusion.
As study conducted on hospitalized haematology-oncology patients demonstrated that although ABO-major mismatched PLT transfusions resulted in decreased post transfusion PLT increment, it did not increase the risk of major haemorragic manifestations [15]. However, an increment in platelet count can be reassuring to the patients and a lack of rise in platelet count after transfusion can lead to undue apprehension.
A randomized trial consisting of 26 haematology-oncology patients found that the incidence of acquiring PLT refractoriness was lower amongst those who received ABO identical PLTs compared to those who received ABO mismatched PLTs [16].
Other studies have demonstrated that there was no difference in the number of PLTs and RBCs transfused, development of fever, length of stay in hospital, or antibiotic use to patients who received ABO identical PLT units compared to those who received ABO-major mismatched PLTs [17,18].
A study by Julmy F et al., also showed that transfusion efficiency of ABO mismatched platelets in children is inferior to that of ABO identical platelets [19].
However, a study by van Eys J et al., demonstrated contrasting findings [20]. A study on cardiovascular surgery patients showed no difference in PLT counts after ABO identical and mismatched transfusions [21].
A study on lymphoma patients, ABO identical PLT transfusions, was associated with fewer units of PLTs and RBCs transfused compared to recipients of ABO mismatched PLTs [22]. In the present study recipients of ABO compatible pooled platelet transfusions received lesser number of platelet transfusions to maintain stable platelet counts (Average of 4units for responders and 8units for non-responders).
Most of the studies performed abroad for the effectiveness of platelet transfusion have utilized apheresis platelet units. However they are extremely expensive and not affordable in case of patients from poor socioeconomic strata. Occasional patients with dengue fever may require multiple transfusions. In this study, pooled platelets were used as they were less expensive and could be transfused a couple of times.
Limitation
As this was a retrospective study, specific tests to check the tires of anti A or anti B in donor plasma, blood group and Human leukocyte antigen (HLA) compatibility of platelets with recipient could not be performed and also equal number of participants could not be considered in the study groups.
Conclusion
Due to various constraints, most transfusion services issue ABO mismatched platelets when type-specific platelet products are not available. The effectiveness of ABO compatible and incompatible pooled platelet transfusion therapy in serologically proven dengue patients was evaluated in this study. Although it was a retrospective study and was limited by the fact that cross matching assays could not be performed on platelet units, it was found that ABO identical pooled platelet transfusion resulted in higher post transfusion platelet count increments. Thus the practice of using ABO identical and ABO compatible platelet units even while using random donor pooled platelets can be life saving. ABO identical and compatible pooled platelet transfusions can also prevent multiple platelet transfusions performed to prevent bleeding risks. Thus it is economical for patients from poor socioeconomic strata and also prevents unnecessary platelet transfusions and wastage of platelet products.