Nanoleakage Evaluation of Posterior Teeth Restored with Low Shrinkable Resin Composite- An invitro Study
Labib Mohamed Labib1, Sameh Mahmoud Nabih2, Kusai Baroudi3
1 Lecturer, Department of Restorative Dental Sciences, Alfarabi Colleges, Riyadh, Saudi Arabia.
2 Professor, Department of Operative Dentistry, Faculty of Dental Medicine, Al–Azhar, University, Cairo, Egypt.
3 Associate Professor, Department of Preventive Dental Sciences, Alfarabi Colleges, Riyadh, Saudi Arabia.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kusai Baroudi, Associate Professor, Department of Preventive Dental Sciences, Alfarabi Colleges, Riyadh, Kingdom of Saudi Arabia.
E-mail: d_kusai@yahoo.co.uk
Introduction
The effect of nanoleakage on the integrity of resin–dentin bond has been in interest for long-term adhesion.
Aim
This study evaluated the nanoleakage in premolar teeth restored with low shrinkable resin composite.
Materials and Methods
A total of 40 human premolars were used for nanoleakage evaluation in this study. Each group was divided into four equal groups; Group A: using silorane with its adhesive system. Group B: using silorane with G-bond. Group C: using Filtek supreme composite with G-bond. Group D: using Filtek supreme composite with AdheSE adhesive. Nanoleakage analysed using Scaning Electron Microscope (SEM) and Energy Dispersive X-Ray Spectrometery (EDX).
Results
The amount of silver present in hybrid layer depend on the adhesive used; this indicated different nanoleakage expressions in different adhesive systems. Filtek Z350 composite with G-bond showed clear silver uptake in both the adhesive and hybrid layer. Low shrinkable resin composite (silorane) with its adhesive system showed less silver penetration and slight silver peak on the elemental energy spectroscopy of energy dispersive X-Ray spectrometry (EDS) as compared to other samples.
Conclusion
Adhesives used between different groups, influence the location and degree of nanoleakage. There is difference in nanoleakage patterns between two-step and one-step adhesives and also among the one-step adhesives themselves.
Filtek supreme, Hybrid layer, Silorane
Introduction
Although the developments that have been made in the field of resin composites for dental applications, shrinkage stresses due to the polymerization process of resin composites continues to be a major problem [1]. The composite shrinkage, creates stresses within the material at the tooth structure interface which may be manifested as nanoleakage which in turn compromises the synergism of the bond at the tooth restoration interface possibly leading to bacterial microleakage and ultimately to marginal discoloration, secondary caries and pulpal inflammation [2,3]. Resin composites applied in restorative dentistry exhibit volumetric shrinkage depending on the formulation and curing condition [4,5]. Silorane dental composite has new ring-opening monomers that modify its resin matrix [6]. This modified resin matrix consists of siloxane and oxirane, thus the name silorane [7]. This new restorative material has less shrinkage stresses and good mechanical properties when compared to those of the methacrylate based composites [8,9]. The silorane adhesive is composed of a hydrophilic one-step self-etch primer and hydrophobic viscous bond coating resin. The manufacturer claims that the one-step self-etch choice is based on the increased popularity of this category of adhesives. However, one-step self-etch adhesives have limitations when bonding to dentin and their long term bond is still undetermined [10–12]. This new composite can form a strong bond with identical material, but cannot form bonds with dissimilar materials. If the silorane composite resin can make a bond with methacrylate-based adhesive, this will improve or at least maintain bond strengths durability and decrease levels of nanoleakage as well [13].
Silver grains penetration is considered a good method for evaluation of nanoleakage due to submicron defects in resin infiltration or inadequate polymerization. However, there is controversial matter between nanoleakage and the quality of resin–dentin bonds [14–16]. This work is an extension of previous work that evaluated the cuspal deflection in premolar teeth restored with low shrinkable resin composite [17].
The purpose of this study was to evaluate nanoleakage in premolar teeth restored with low shrinkable resin composite.
Materials and Methods
The study was an invitro study conducted in Al-Azhar University for two years. Forty human premolars extracted for orthodontic reasons and stored in normal saline were used. Patients consent was obtained. This study was approved by Al-Azhar University (under number 490-Part b/2013). Crown segments were obtained by first removing the roots 1mm beneath the cemento–enamel junction using a slow-speed water-cooled diamond disk. The entire specimen except for the bonded interface and 1mm of the tooth bordering adjacent to the interface, was coated with two layers of nail varnish. The specimens were placed in a 50% (w/v) silver nitrate solution (pH=9.5) in total darkness for 24 h, the perfusion procedure was carried out in a dark room in order to avoid artifacts of pseudo staining and/or overstraining, then rinsed in running water for 5 min, immersed in photo developing solution, and exposed to a fluorescent light for 8 h in order to reduce the silver ions to metallic silver.
The specimens were placed in running water for 5 min after removal from the developing solution. The resin–dentin end of the assembly was cut into multiple parallel slabs, then after rotating the assembly about 90°, another series of parallel cuts were made. To remove the resin–dentin sticks from the acrylic cube a final cut was performed within the composite buildup resulting in resin–dentin sticks with a dimension of 1mm×2mm and a length of 6mm–8mm.
The specimens were divided into two main equal groups then further subdivided in to four equal subgroups. Subgroup A: using silorane with its adhesive system. Subgroup B: using low silorane with G-bond. Subgroup C: using filtek supreme composite with G-bond. Subgroup D: using Filtek supreme composite with AdheSE adhesives. The side of dentin sticks was analyzed in a field emission SEM; also analyzed using EDX spectrometry. Comparison between the four different subgroups was made using four-dimensional mapping which was performed over 50mm X 50mm areas across the adhesive–dentine interface, these areas covered the adhesive layer, the Hybrid Layer (HL), partially demineralized and un-affected dentine and were visualized and focused at 1200X magnification. Amount of silver (Ag) grains that penetrated at resin-dentin interface was calculated and statistically analyzed through energy levels of EDX analysis.
Results
The silver nitrate penetration method, combined with high magnification SEM by means of secondary electron or backscattered electron mode, can provide much better information concerning the sealing ability of the restorations and the quality of the hybrid layer.
In this study a comparison was performed between four groups and their corresponding adhesive system by the use of EDX spectrometery spectrum that recorded the amount of silver grains (wt%) presented in area (50-50μ) at resin dentin interface and demonstrated it by silver uptake (Ag) peaks. A thin layer of resin composite was used to avoid the possible effects of polymerization shrinkage. The length of silver penetration along the interface was not recorded.
The results in this study demonstrated different leakage patterns depending on the dentin bonding systems tested. However, silver ion accumulations were often noted at the base of the hybrid layer for all materials. Silorane with its adhesives, Group A [Table/Fig-1a,b] exhibited little amount of nanoleakage, with smallest peak of Ag (5.42 wt%) [Table/Fig-2].
Backscattered electron image of SEM (a) and corresponding EDX spectrum (b) of the fractured surface of the dentin side of specimen (Group A).
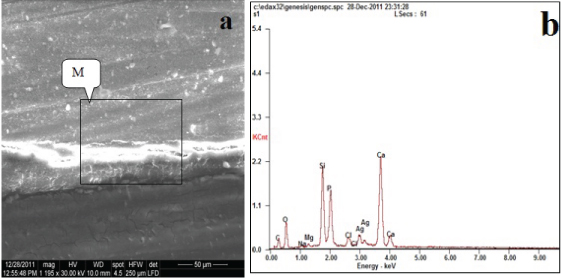
Amount of silver in different groups at energy level (L). The level that represent the amount of silver grains in energy dispersive X-ray spectrometey (EDX) during analysis.
| Groups | Elemnt | Amount of silver by wt% | Energy level |
|---|
| Group A | Ag | 05.42 | L |
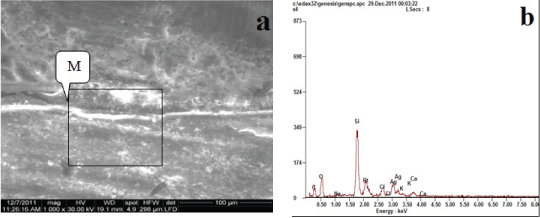
| Group B | Ag | 09.19 | L |
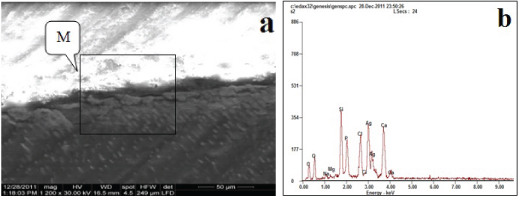
| Group C | Ag | 19.10 | L |
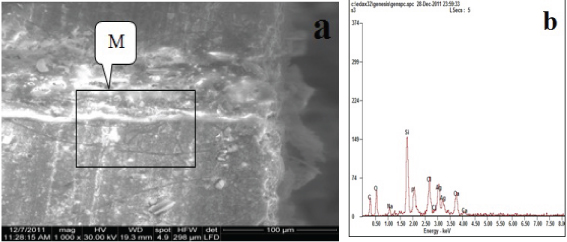
| Group D | Ag | 12.98 | L |
Regarding Group B: Silorane with G-bond adhesive [Table/Fig-3]; it was found that the granules of silver depositions on the top of the hybrid layer, showed that silver uptake was very low, this accommodated with amount of silver (9.19 wt%) in [Table/Fig-2]. The EDX spectrum of the granules demonstrated by a box in [Table/Fig-3a] identified those granules as silver in [Table/Fig-3b] by the sharp Ag peak.
Backscattered electron image of SEM (a) and corresponding EDX spectrum (b) of the fractured surface of the dentin side of specimen (Group B).
Regarding Group C: Methacrylate with G-bond adhesive, the granules of silver depositions (at the marked M) on the top of the hybrid layer, showed that silver uptake was very high, when compared with the other groups this accommodated with amount of silver (19.10 wt%) in [Table/Fig-2]. The EDX spectrum of the granules demonstrated by a box in [Table/Fig-4a] identified those granules as silver in [Table/Fig-4b] by the sharp Ag peak.
Backscattered electron image of SEM (a) and corresponding EDX spectrum (b) of the fractured surface of the dentin side of specimen (Group C).
Regarding Group D: Methacrylate with AdheSE adhesive. The granules of silver depositions on the top of the hybrid layer showed that silver uptake was low, this accommodated with amount of silver (12.98 wt%) in table [Table/Fig-2]. The EDX spectrum of the granules demonstrated by a box in [Table/Fig-5a] identified those granules as silver in [Table/Fig-5b] by the sharp Ag peak.
Backscattered electron image of SEM (a) and corresponding EDX spectrum (b) of the fractured surface of the dentin side of specimen (Group D).
Discussion
In this study a comparison was done between four groups and their corresponding adhesive system by the use of Energy-dispersed X-ray (EDX) spectrum that record the amount of silver grains (wt%) present in area (50-50 m) at resin dentin interface and demonstrate it by Ag peaks [18,19]. A thin layer of resin composite was used to avoid the possible effects of polymerization shrinkage. The length of silver penetration along the interface was not recorded, since this study was focused to determine if a variation in the nanoleakage patterns occurred among the materials tested.
Silorane with its adhesives (Group A) exhibit little amount of nanoleakage, with smallest peak of Ag and the amount of Ag was the smallest one (5.42 wt%) compared to the other samples, this may be explained by the fact that siloranes are a new class of resin composites that are made up of various combinations of siloxanes and oxiranes. Siloxanes are derivatives of silicone and oxygen. When the oxirane ring opens there is less shrinkage than when methacrylate double bonds are converted to single bonds. The reason siloxanes were created was to reduce polymerization shrinkage and stress [20] and when they are mixed with oxiranes, they become siloranes. The silorane backbone is very hydrophobic. Oxiranes are also hydrophobic. It was thought that if oxiranes (i.e., epoxides) are exposed to water, the epoxide ring would open and interfere with polymer chain growth. However, a recent study concluded that 3,4-epoxy-clohexylethylcyclopolymethylsiloxane (Tet-Sil), a likely monomer in silorane, was stable when incubated in aqueous solutions [21,22]. The oxirane groups were not hydrolyzed because the monomer was immiscible in water [23]. That is, water could not reach the oxirane groups. Silorane is a two-step adhesive system. The first step involves placing a self-etching primer on the smear layer covered dentine. This is sufficiently acidic to etch away most of the smear layer without removing all of the smear plugs or peritubular dentine. It also demineralizes the intertubular dentine to a depth of 1–1.5μ. After evaporating the solvent, the hydrophilic primer is light cured. This primer is designed to seal the dentine and convert it from a wet hydrophilic, collagenous surface, to a dry hydrophobic surface that can couple with the silorane adhesive [24]. Dentin primer was covered with a thick layer of a very hydrophobic adhesive that did not take up any silver nitrate. The hydrophobic nature of the silorane adhesive is manifested as a lack of water (i.e., silver) diffusion these have been shown to exhibit little nanoleakage. Methacrylate (Z350)with G-bond (Group C) exhibit the largest amount of nanoleakage, largest peak of Ag and amount of Ag was the largest one (19.14 wt%) compared to the other samples this may be explained by the fact that the all-in-one adhesive, G-Bond is a single-bottle self-etching adhesive that contains both 4-MET, a carboxylic acid methacrylate derivative and a phosphoric acid ester of methacrylate, along with a dimethacrylate, dissolved in water/acetone with a pH of 2. As it is HEMA-free, when the acetone begins to evaporate more rapidly than the water, the water coalesces into 2–10μm round droplets in the adhesive [25]. In the current study, silver nitrate accumulated in these droplets. In contrast to the free water droplets in the overlying adhesive layer that probably represent residual water from the adhesive, the interfacial blister-like water presumably seeped into the interface from the tubules during bonding [26,27]. The larger size of the silver grains in bonded dentine interfaces created by G-Bond, relative to the other adhesives used in this study, might be a result of that adhesive system exhibiting larger water droplets. Methacrylate (Z350) with AdheSe adhesive (Group D) exhibit large amount of nanoleakage, large peak of Ag and amount of Ag was large (12.98 w%) but less than amount showed in (Group C) this is due to the fact that dimethacrylates are hydrolytically unstable in acidic aqueous solutions of one-step self-etch adhesives, which may result in poorer cross-linking, lower bond strengths and compromised bond durability due to great amount of nanoleakage. Therefore, AdheSe adhesive that have acrylamides which already contains monofunctional and cross-linking monomers have been developed, to increase hydrolytical stability [27].
The evaluation of the clinical significance of nanoleakage is an important procedure, further more development of adhesive systems minimizes nanoleakage to optimize dentin bonding. But the influences in marginal discoloration, recurrent caries, post-operative symptoms and the longevity of the composite restoration cannot be excluded.
Limitation
The nanoleakage measurements might be done by two methods to compare the results and validate the methodology used.
Conclusion
The location and degree of nanoleakage, depends on the adhesives used between different groups that are utilized. The present study revealed that there is a difference in nanoleakage expressions between two-step and one-step self-etch adhesives.
[1]. Schneider L, Cavalcante L, Silikas N, Shrinkage stresses generated during resin-composite applicationJ Dent Biomech 2010 2010:131630 [Google Scholar]
[2]. Cara R, Fleming G, Palin W, Walmsley A, Burke F, Cuspal deflection and microleakage in premolar teeth rest ored with resin-based composites with and without an intermediary flowable layerJ Dent 2007 35:482-89. [Google Scholar]
[3]. Kleverlaan C, Feilzer A, Polymerization shrinkage and contraction stress of dental resin compositesDent Mater 2005 21:1150-57. [Google Scholar]
[4]. Weinmann W, Thalacker C, Guggenberger R, Siloranes in dental compositesDent Mater 2005 21:68-74. [Google Scholar]
[5]. Guggenberger R, Weinmann W, Exploring beyond methacrylatesAm J Dent 2000 13:82D-84D. [Google Scholar]
[6]. Bouillaguet S, Gamba J, Forchelet J, Krejci I, Wataha JC, Dynamics of composite polymerization mediates the development of cusplstrainDent Mater 2006 22:896-902. [Google Scholar]
[7]. Ilie N, Hickel R, Silorane-based dental composite: behavior and abilitiesDent Mater J 2006 25:445-54. [Google Scholar]
[8]. Frankenberger R, Tay F, Self-etch vs. etch-and-rinse adhesives: effect of thermo-mechanical fatigue loading on marginal quality of bonded resin composite restorationsDent Mater 2005 21:397-412. [Google Scholar]
[9]. Mitsui F, Peris A, Cavalcanti A, Marchi G, Pimenta L, Influence of thermal and mechanical load cycling on microtensile bond strengths of total and self-etching adhesive systemsOper Dent 2006 31:240-47. [Google Scholar]
[10]. Duarte S, Phark J, Mansur F, Sadan A, Nanoleakage, ultramorphological characteristics, and microtensile bond strengths of a new low-shrinkage composite to dentin after artificial agingDent Mater 2009 25:589-600. [Google Scholar]
[11]. Okuda M, Pereira PNR, Nakajima M, Tagami J, Pashley DH, Long-term durability of resin dentin interface: nanoleakage vs. microtensile bond strengthOper Dent 2002 27:289-96. [Google Scholar]
[12]. Sauro S, Pashley D, Mannocci F, Tay F, Pilecki P, Sheriff M, Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using a double-staining/confocal microscopy techniqueEur J Oral Sci 2008 116:184-93. [Google Scholar]
[13]. Hashimoto M, Munck J, Ito S, Sano H, Kaga M, Oguchi H, In vitro effect of nanoleakage expression on resin-dentin bond strengths analyzed by microtensile bond test, SEM/EDX and TEMBio Mater 2004 25:5565-74. [Google Scholar]
[14]. Sano H, Takatsu T, Ciucchi B, Horner J, Matthews W, Pashley D, Nanoleakage: leakage within the hybrid layerOper Dent 1995 20:18-25. [Google Scholar]
[15]. Sano H, Shono T, Takatsu T, Hosoda T, Microporous dentin zone beneath resin impregnated layerOper Dent 1994 19:59-64. [Google Scholar]
[16]. Wu W, Cobb E, Dermann K, Detecting margin leakage of dental composite restorationsJ Biomed Mater Res 1983 17:37-43. [Google Scholar]
[17]. Labib LM, Nabih SM, Baroudi K, Evaluation of cuspal deflection in premolar teeth restored with low shrinkable resin composite (in vitro study)J Int Soc Prev Community Dent 2015 5:470-75. [Google Scholar]
[18]. Inoue S, Peumans M, Suzuki K, Lambrechts P, Van B, Monomer–solvent phase separation in one-step self-etch adhesivesJ Dent Res 2005 84:183-88. [Google Scholar]
[19]. Van K, Snauwaert J, Munck J, Coutinho E, Poitevin A, Yoshida Y, Origin of interfacial droplets with one-stepadhesivesJ Dent Res 2007 86:739-44. [Google Scholar]
[20]. Moszner N, Fischer U, Angermann J, Rheinberger V, Bis-(acrylamide)s as new cross- linkers for resin-basedcomposite restorativesDent Mater 2006 22:1157-62. [Google Scholar]
[21]. Santini A, Miletic V, Comparison of the hybrid layer formed by silorane adhesive, one-step self-etch and etch and rinse systems using confocal micro-Raman spectroscopy and SEMJ Dent 2008 36:683-91. [Google Scholar]
[22]. Fleming G, Cara R, Palin W, Burke F, Cuspal movement and microleakage in premolar teeth restored with resin-based filling materials cured using a ‘soft-start’ polymerisation protocolDent Mater 2007 23:637-43. [Google Scholar]
[23]. Palin W, Fleming G, Burke F, Marquis P, Randall R, The influence of short and medium-term water immersion on the hydrolytic stability of novel low-shrink dental compositesDent Mater 2005 21:852-63. [Google Scholar]
[24]. Eick J, Smith R, Pinzino C, Kostoryz E, Stability of silorane dental monomers in aqueous systemsJ Dent 2006 34:405-10. [Google Scholar]
[25]. Van Meerbeek B, Perdigao J, Lambrechts P, Vanherle G, The clinical performance of adhesivesJ Dent 1998 26:1-20. [Google Scholar]
[26]. Spencer P, Wang Y, Adhesive phase separation at the dentin interface under wet bonding conditionsJ Biomed Mater Res 2002 62:447-56. [Google Scholar]
[27]. Tay F, Gwinnett A, Wei S, Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free, acetone-based, single-primer/adhesivesDent Mater 1996 12:236-44. [Google Scholar]