From the stand point of radiographic analysis most of the complex cyanotic congenital heart diseases (CHD), can be divided into those associated with decreased or increased pulmonary vascularity. Combination of a specific cardiac configuration and status of lung vasculature in a clinical context allows plain film diagnosis to be predicted in some CHD. Correlation of the position of the cardiac apex in relation to the visceral situs is an important information that can be obtained from the plain film. This information helps in gathering information about the atrio-ventricular, ventricular arterial concordance or discordance. Categorization of the cyanotic heart disease based on vascularity is presented below. Thorough understanding of cardiac anatomy by different imaging methods is essential in understanding and interpreting complex cardiac disease. Basic anatomical details and background for interpretation are provided in the previous parts of this presentation.
Ebsteins anomaly, Echocardiography, Cardiac imaging, Pulmonary atresia, Tetralogy, Transposition of great arteries, Truncus arteriosus
Complex Congenital Heart Disease
Evaluation of complex congenital heart disease (CHD) demands obtaining detailed and specific information regarding cardiac chambers, connections, muscle contractility and quantification of the flow across valves, septal defects and collaterals. Plain radiography remains starting point and mainstay as a global assessment tool in pre and postoperative evaluation of the patients. Echocardiography initiates detailed investigative process, guided by clinical questions. If additional information is needed [1–4], MRI provides most precise information required for preoperative and post-operative evaluation for CHD. MDCT is a valid alternative with some limitations (inadequate functional information, radiation dose and contrast related complications), essentially providing structural information necessary for surgical management. CT evaluation also provides details about pulmonary anomalies, structural information regarding lungs, secondary complications and evaluation of tracheo-bronchial compression. CT also provides detailed mapping of collateral vasculature, information about postoperative status of surgical procedures, grafts and bony changes in association with CHD [5–8]. Integrated multimodality approach is required for, the management of the complex CHD, often decided in consultation with a multidisciplinary team consisting of paediatric cardiologist, cardiac surgeon and an imaging specialist.
Following commonly encountered entities are described in detail [Table/Fig-1]
Cyanotic congenital heart disease [9].
| Increased Pulmonary Vascularity | Decreased Pulmonary Vascularity |
|---|
| Total anomalous pulmonary venous connection (TAPVC) | Tetralogy of Fallot |
| Truncus arteriosus | Tricuspid atresia |
| Transposition of great arteries | Pulmonary atresia |
| Single ventricle | Pulmonary stenosis and atrial septic defect |
| Double outlet right ventricle | Ebstein anomaly |
Tetralogy of Fallot (TOF) and variants,
Pulmonary Artesia- (pseudo-Truncus),
Double Outlet Right Ventricle (DORV),
Transposition of Great Arteries (TGA),
Truncus Arteriosus (TA),
Total Anomalous Pulmonary Venous Connections (TAPVC):
(a) Supracardiac; (b) Intracardiac; and (c) Infracardiac varieties.
Ebsteins anomaly.
Tetralogy of Fallot (TOF) [Table/Fig-2]: Tetralogy of Fallot (TOF) [Table/Fig-3a&b,4] is the most common congenital cyanotic heart disease, beyond the neonatal period, accounts for 6-10% [9]. Predominant pathology of TOF is the outflow narrowing of the right ventricle and varying degree of the narrowing of pulmonary artery and branches. Other components include ventricular septal defect and overriding of aorta. Plain radiography typically shows upturned cardiac apex due to right ventricular hypertrophy. (Coeur en Sabot cardiac configuration) Lungs show varying degree of oligemia reflecting the extent of pulmonary outflow obstruction. Prominent right aortic arch is noted in up to 25% of patients. Reconstitution of a pulmonary vascularity is achieved by systemic collaterals and left to right shunting at the ductal level. The goal of surgical procedures in TOF is to increase the capacity of the right ventricular outflow by surgical correction and increase pulmonary flow. Palliation in the form of systemic to pulmonary shunt is performed for increasing the capacity of pulmonary arteries in staged procedures. Imaging requirement is to demonstrate the extent and severity of narrowing of pulmonary outflow and arteries, demonstration of associated anomalies and showing extent of pulmonary atresia or discontinuity. Measurement of right ventricular volume is very critical for staged reconstruction of outflow. MRI is the procedure of choice for functional assessment although MDCT offers accurate anatomical information in this regard [10]. Evaluation of coronary artery anomaly, exclusion of large conus branch in the region of outflow and demonstration of anomalous right coronary from LAD artery can be done by CT angiography [11]. Various measurable indices and ratio are described in the following section to provide a quantitative measure of size of pulmonary artery and branches. Quantitative information regarding size of pulmonary artery will help in deciding required stages of corrective procedure. Pulmonary atresia with VSD [Table/Fig-5a&b] is a variant of TOF, due to complete atresia of the pulmonary artery. Surgical procedures are more complex in patients with large atretic segment. Role of MDCT is well established in pre-operative assessment [12]. Unusually large systemic collateral arteries may be noted, occasionally with bronchial impression [Table/Fig-6a,b and c]. In a patient with TOF with absent pulmonary valves, there is associated pulmonary regurgitation and severe dilatation of the branches of pulmonary arteries. Dilated arteries can cause pressure effect on adjacent airways and cause secondary respiratory symptoms [Table/Fig-7]. Phase contrast MR techniques allow measurement of regurgitant flow; constitute part of pre and postoperative assessment.
| TOF | |
|---|
| Components | VSD (usually perimembranous) | RVOT obstruction (at infundibular, pulmonary valvular or combination) | RVH | Overriding of aorta |
| Incidence [13] | 1-3 cases per 1000 live births. Most common cyanotic CHD.Arises from single gene defect involving TBX1 gene (TBX1 gene encodes for a transcriptional factor integral to development of cardiac outflow tracts) |
| Association [13] | Right aortic arch (25%). Abnormal coronary arteries (5%) LAD arising from RCA with prepulmonic course being most common. Complete AVSD (2%) Persistent left SVC |
| Syndromes | CHARGE syndrome Di George syndrome Shprintzen (velo-cardio-facial) syndrome |
| Imaging features Plain | Boot shaped heart (cor en Sabot)= concave MPA segment with an upturned apex | Pulmonary oligemia | Thymic atrophy | Right aortic arch |
| Imaging features specific | Echo defines all components of TOF MRI useful in post-surgical follow-up for RV volume assessment, pulmonary regurgitation quantification and to rule out residual VSD CT evaluation of pulmonary arteries, collaterals and coronary arteries* McGoon Ratio and Nakata Index are used for quantification of degree of PA hypoplasia |
| Management [13] | Palliative procedures:• Modified Blalock-Taussig shunt most preferred; placement of Gore-Tex interposition graft between subclavian artery and ipsilateral pulmonary artery Definitive surgery: Total ICR (intracardiac repair) VSD patch closure + widening of RVOT (infundibular tissue resection) + pulmonary valvotomy Note: Presence of coronary anomalies (esp. LAD from RCA with RVOT crossing) is a contraindication for primary repair. |
TOF Severe outflow obstruction.
A three-month-old female presented with cyanosis on feeding, diagnosed as Tetralogy on echocardiography, confirmed on MDCT examination; (a) Diagrammatic representation of cardiac anatomy in TOF; (b) Plain radiograph shows moderate cardiac enlargement with upturned apex (RV configuration) (triangle) and decreased pulmonary arterial markings; (c) Echocardiography long axis view demonstrates a large malaligned perimembraneous VSD (arrow); (d) Colour Doppler examination of RV outflow demonstrates narrowing of pulmonary artery with turbulent flow.
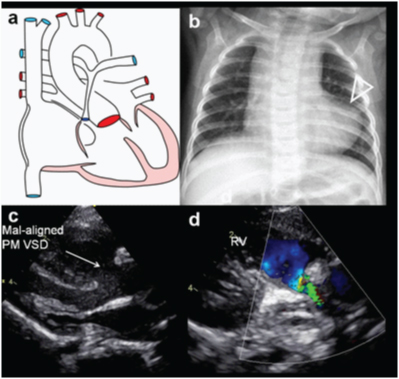
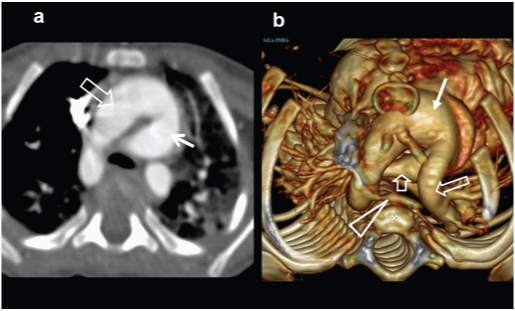
A three-month-old female presented with cyanosis on feeding, diagnosed as Tetralogy on echocardiography, confirmed on MDCT examination: (e) axial CT images shows a large subaortic ventricular septal defect (arrow) and RVH; (f) Sagittal CT demonstrates large aorta(triangle) with normal branching pattern; (g) axial CT image at a slightly higher level shows severe narrowing of pulmonary infundibulum and proximal pulmonary artery (arrow). Right and left pulmonary arteries are relatively small. Also, there are enlarged collateral arteries in chest wall (open arrow) (h) 3D reconstruction of aorta shows enlarged major aorto-pulmonary collateral arteries (MAPCA) (arrows).
TOF Moderate outflow obstruction.
A 21-year-old female presented with palpitations, right ventricular outflow obstruction on echocardiography diagnosed with PS; (a) Diagrammatic representation of cardiac anatomy in TOF (b) Plain radiograph shows normal cardiac size and configuration. Pulmonary arterial markings are decreased; (c) RVOT reconstructed CT; and (d) axial CT image at a slightly higher level shows severe narrowing of pulmonary infundibulum, pulmonary valve and proximal PA (arrows). RPA and LPA are relatively small; (e) Reconstructed CT view shows a large sub-aortic VSD (arrow) and large overriding aorta. There is biventricular enlargement and hypertrophy.
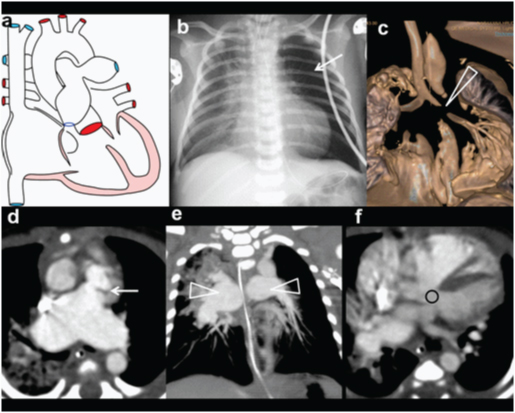
Pulmonary Atresia.
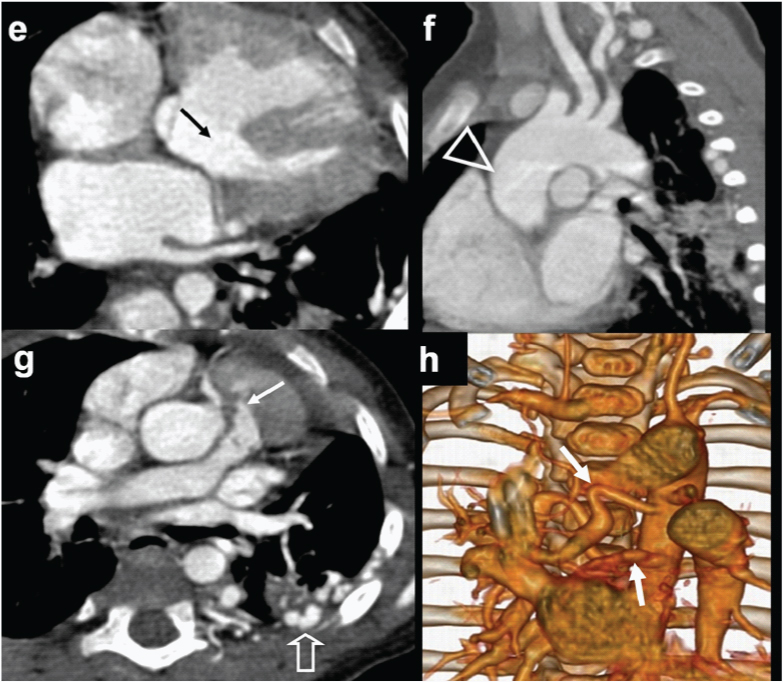
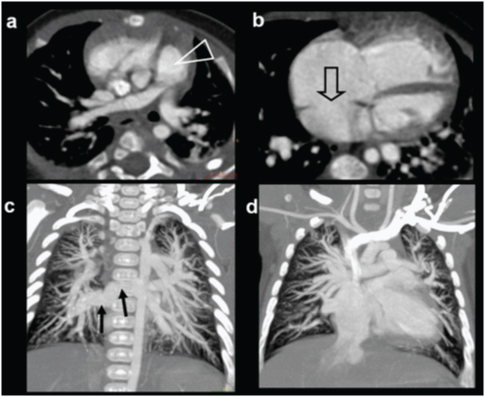
An eight-month-old male with cyanosis at birth, feeding difficulty; failure to demonstrate pulmonary artery on echocardiography diagnosed with pulmonary atresia: (a) Diagrammatic representation of cardio-vascular anatomy in pulmonary atresia; (b) Plain radiograph shows mild cardiac enlargement with upturned apex (RV configuration) and decreased pulmonary arterial markings. Collateral vessel around right hilar region are prominent (open arrow); (c) axial CT images shows a large sub-aortic ventricular septal defect (arrow) and RVH; (d) Reconstructed outflow CT view shows overriding of aorta and large sub-aortic VSD (arrow).
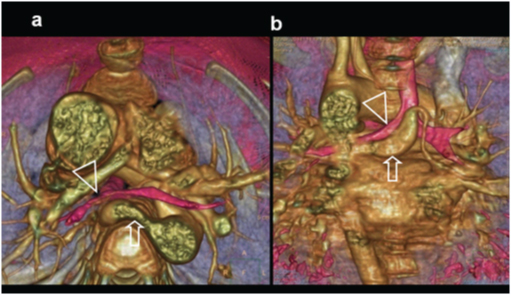
An eight-month-old male with cyanosis at birth, feeding difficulty and failure to demonstrate pulmonary artery on echocardiography: Diagnosed with pulmonary atresia: (e) Axial CT slightly at a lower level shows blind ending pulmonary infundibulum (angled arrow). Main pulmonary artery is absent. Right and left pulmonary arteries reconstituted by collaterals (triangles); (f) 3D reconstruction of aorta shows enlarged collateral arteries (arrow) (MAPCA).
Pulmonary atresia with bronchial compression by collateral artery.
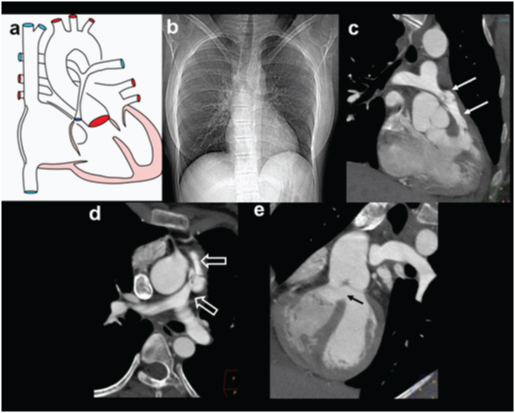
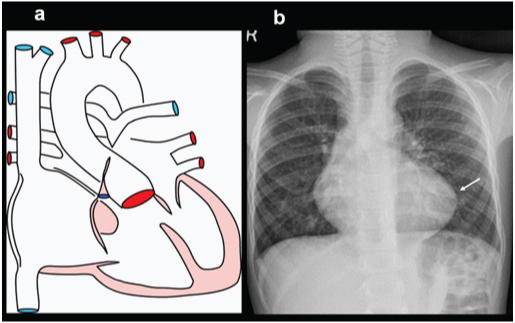
A three-year-old female presenting with exertional dyspnoea, diagnosed with a pulmonary atresia on echocardiography: (a) Diagrammatic representation of vascular anatomy in Pulmonary atresia; (b) Plain radiograph shows mild cardiac enlargement with upturned apex(arrow) (RV configuration) and decreased pulmonary arterial markings.
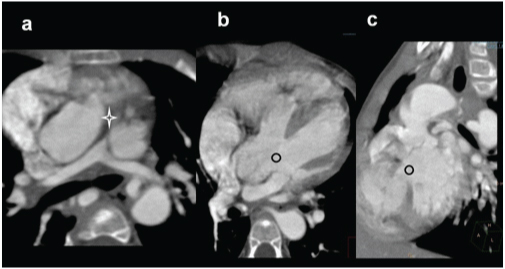
A three-year-old female presenting with exertional dyspnoea, diagnosed with a pulmonary atresia on echocardiography: (a) axial CT images shows a atretic main pulmonary artery (star) and small right and left pulmonary arteries; (b,c) Reconstructed outflow CT views shows overriding of aorta and large sub-aortic VSD(circles).
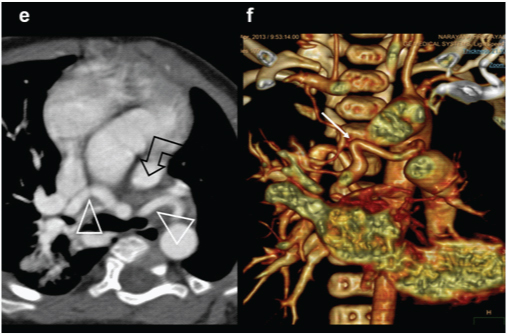
A three-year-old female presenting with exertional dyspnoea, diagnosed with a pulmonary atresia on echocardiography: (a) 3D reconstruction of aorta and airways (red) shows anatomy of pulmonary arteries, enlarged collateral arteries (MAPCA) (open arrows) impressing on the airways (triangle); (b).
TOF absent Pulmonary valves: A One-day-old female child presenting with respiratory distress, cyanosis, diagnosed with TOF with absent pulmonary valve on echo and CT: (a) Diagrammatic representation of vascular anatomy in TOF with absent pulmonary valve: (b) Plain radiograph shows hyperinflated left lung (arrow) due to partial left bronchial compression; (c) Coronal 3-D airway reconstruction demonstrates left bronchial compression(triangle); (d) Axial MIP CT image shows gross dilatation of pulmonary arteries with relative narrowing at valve level (arrow); (e) CT coronal MIP view shows severe dilatation of RPA and LPA (triangles); (f) Axial CT images reveals subaortic VSD (circle).
McGoon Ratio = diameters of distal RPA+ distal LPA (immediately pre-branching portions)/ aortic diameter just above diaphragm {normal ratio: 2.0-2.5}.
Nakata Index = cross sectional areas of RPA and LPA (in mm2)/ BSA {normal= 330 mm2/BSA}.
Details regarding variants of TOF, namely Pulmonary Atresia: [Table/Fig-8a&b] and absent pulmonary valve are provided in tables [Table/Fig-9].
Pulmonary atresia PA-IVS.
| Subtypes | PA-IVS (Pulmonary atresia with intact ventricular septum) | PA-VSD (Pulmonary atresia with VSD) |
|---|
| PA-IVS (Hypoplastic right heart syndrome) Characterized by pulmonary atresia, hypoplastic RV and hypoplasia of tricuspid valve annulus |
| Incidence [13] | 7.1-8.1 per 100,000 live births |
| Association | Coronary artery anomalies e.g. RV dependent coronary circulation (coronary circulation perfused entirely by desaturated RV blood with proximal coronary arteries), coronary ostial atresia ASD/PFO PDA. |
| Syndromes | Di George syndrome |
| Imaging features Plain | Normal or mild cardiomegaly | Pulmonary oligemia | Hyper translucent lung fields |
| Imaging features specific | Echo thick, immobile atretic pulmonary valve; smallish hypertrophied RV; small tricuspid valve; ASD and PDACatheter angiography for coronary anomalies and hemodynamic assessmentMRI depicts all findings including tricuspid valve and RV sizeCT preferred for coronary artery evaluationImaging is useful in classifying pulmonary atresia based on presence or absence of 3 portions (inlet/trabecular/infundibular) of RV into tripartite, bipartite and monopartite types [13]. |
| Management [13] | Medical: Start PGE1 to maintain ductal patencySurgical: depends on RV size and coronary artery anomalies
Two-ventricular repair adequate RV size and RVOT necessary One and one-half ventricular repair in borderline RV size One ventricular repair (Fontan operation) in monopartite RV and/or RV dependent coronary circulation.
|
| Frequency [13] | 2.5-3.4% of all CHD. M>F. |
| Association | ASD/PFO Origin of aorta from RV |
| Syndromes | Velo-cardio-facial syndrome DiGeorge syndrome |
| Imaging features Plain | Normal or mild cardiomegaly (Boot shaped heart) | Pulmonary oligemia | Hyper translucent lung fields |
| Imaging features specific | Echo mal-aligned VSD with overriding of aorta, pulmonary atresia MRI can depict all findings CT preferred for coronary artery anomalies and collaterals |
| Management | Medical: Start PGE1 to maintain ductal patencySurgical:
Central shunt surgery: direct connection of ascending aorta and hypoplastic MPA RV-to-PA connection
|
TOF with absent pulmonary valve.
| Components [13] | TOF components + rudimentary nubbins of tissue or complete absence of pulmonary valvular tissueAbsent pulmonary valves, severe PR aneurismal dilatation of MPA and branch pulmonary arteries airway compression. Rabinovitch et al described abnormal tufts of smaller pulmonary arteries that compress the intrapulmonary bronchi with reduction of the number of alveoli. |
| Prevalence | Seen in ~3% of patients with TOF. |
| Association | Frequent absence of ductus arteriosus, Increased nuchal fold thickness (NFT) in I trimester [14] |
| Syndromes | Chromosomal abnormalities of chromosomes 6 and 7; deletion of chromosome 22q11 and DiGeorge syndrome |
| Imaging features Plain | Cardiomegaly (moderate to marked) | Grossly dilated central PAs | Decreased peripheral pulmonary vasculature | Tracheal compression | Air trapping in lungs |
| Imaging features specific | Echo TOF findings+ dysplastic pulmonary annulus+ massive PR on dopplerFetal MRI PA size+ symmetry of aeration of lungs secondary to airway compression and over-inflation + lung volume estimation [15,16]Post-natal MRI PR quantification CT better evaluation of airway compression and secondary lung changes. |
| Management [7] | Complete primary repair= VSD closure + pulmonary homograft placement to replace dysplastic pulmonary valve and dilated PAs |
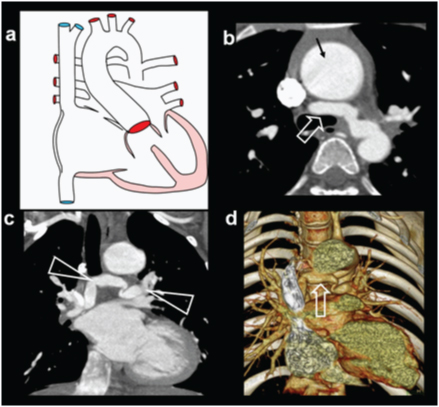
Pseudotruncus: Pseudotruncus refers to severe form of TOF in which pulmonary artery and their proximal branches are completely atretic, disconnecting right ventricles from pulmonary artery [Table/Fig-10]. Large collateral arteries from the aorta provide blood-flow to pulmonary circulation by reconstituting the more distal pulmonary branches.
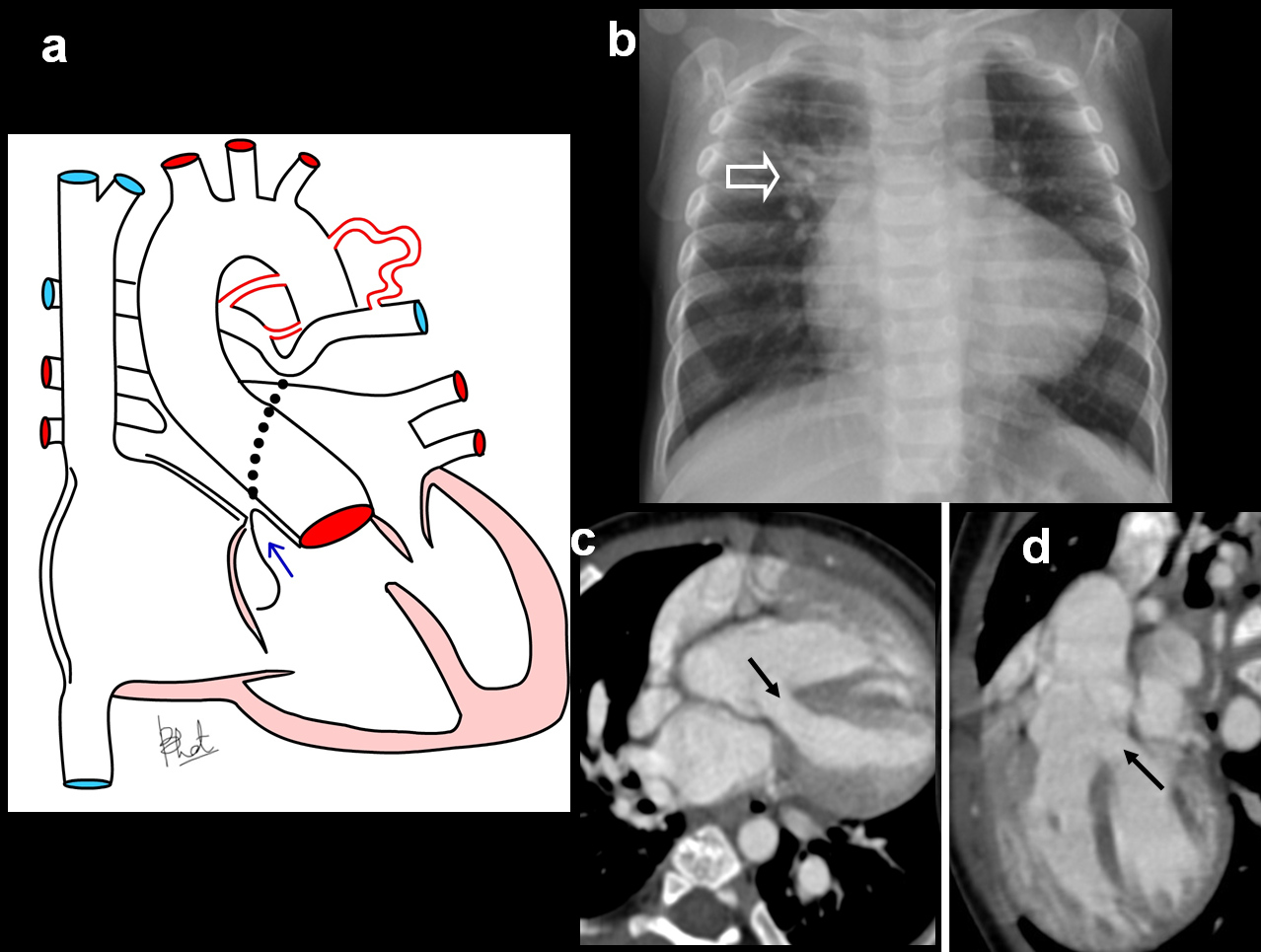
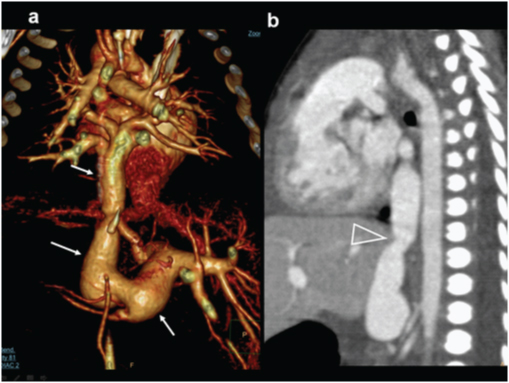
Pseudotruncus. A 10-year-male patient presenting with exertional dyspnoea, investigated for cardiac disease diagnosed with severe pulmonary atresia: (a) Diagrammatic representation patent of vascular anatomy in severe pulmonary atresia (pseudo-truncus); (b) axial CT images shows a large ascending aorta (arrow) and absent pulmonary artery. Large collateral artery is seen arising from descending aorta (open arrow); (c) Reconstructed coronal CT view shows collateral arteries reconstituting pulmonary arteries.(triangles); (d) 3D reconstruction of aorta shows enlarged collateral arteries (open arrow) (MAPCA).
Double Outlet Right Ventricle (DORV) [Table/Fig-11]: DORV is a cono-truncal defect characterized by origin of both great arteries predominantly from RV [Table/Fig-12]. A ventricular septal defect is invariably present; its location in relation to the semilunar valves may be subaortic (50%), subpulmonary (30%), uncommitted, or remote [17]. A large majority (66%) of patients with DORV have some degree of pulmonary stenosis or atresia. DORV has a complex spectrum of physiology based on the location of VSD and flow of blood from ventricle to great arteries. Surgical planning depends upon complexity of physiology, associated anomalies [Table/Fig-13] and whether or not there is pulmonary arterial hypertension [18]. CT imaging with 3D reconstruction of the septum and ventricular outflow provides accurate characterization of types of VSD with good correlation with surgical observation and autopsy [19].
| DORV [13] | Characterized by origin of both great arteries predominantly from RV, bilateral muscular infundibula and absence of AV-semilunar valve continuity. |
| 4 Subtypes (based on location of VSD in relation to great arteries) | DORV with subaortic VSD blood from LV flows via VSD to aorta and blood from RV flows mainly to PA= physiology similar to VSDDORV with subpulmonic VSD (Taussig-Bing syndrome) blood from LV flows via VSD to PA and blood from RV flows mainly to aorta= physiology similar to TGADORV with doubly committed VSD absent infundibular septumDORV with non-committed VSD, VSD remote from aortic and pulmonary valves |
| Incidence | 1-3% of all CHD. M:F ratio=2:1 |
| Association | Coarctation of aortaInterrupted aortic archPulmonary obstruction |
| Syndromes | Trisomy 13, 18Deletion 22q11 |
| Imaging features Plain | Without pulmonary obstruction | Moderate cardiomegaly (RV type), convex MPA, pulmonary plethora, thymic atrophy and hyperinflated lungs |
| With pulmonary obstruction | Mild cardiomegaly (RV type), concave MPA segment, pulmonary oligemia and left arch |
| Imaging features specific | Echo side by side orientation of great vessels with aorta anterior and to the right; overriding of aorta; absent fibrous continuity of semilunar and AV valves MRI very helpful for localization and relation of VSD to great arteries CT. |
| Management [13] | Palliative:
Pulmonary artery banding for uncontrollable CHF in infancy Balloon atrial septostomy for Taussig-Bing anomaly Systemic-to-PA shunting- in pts with associated PS
Definitive repair: Total correction= VSD closure with placement of internal and external conduits to establish physiological blood flow between LV/aorta and RV/PA. |
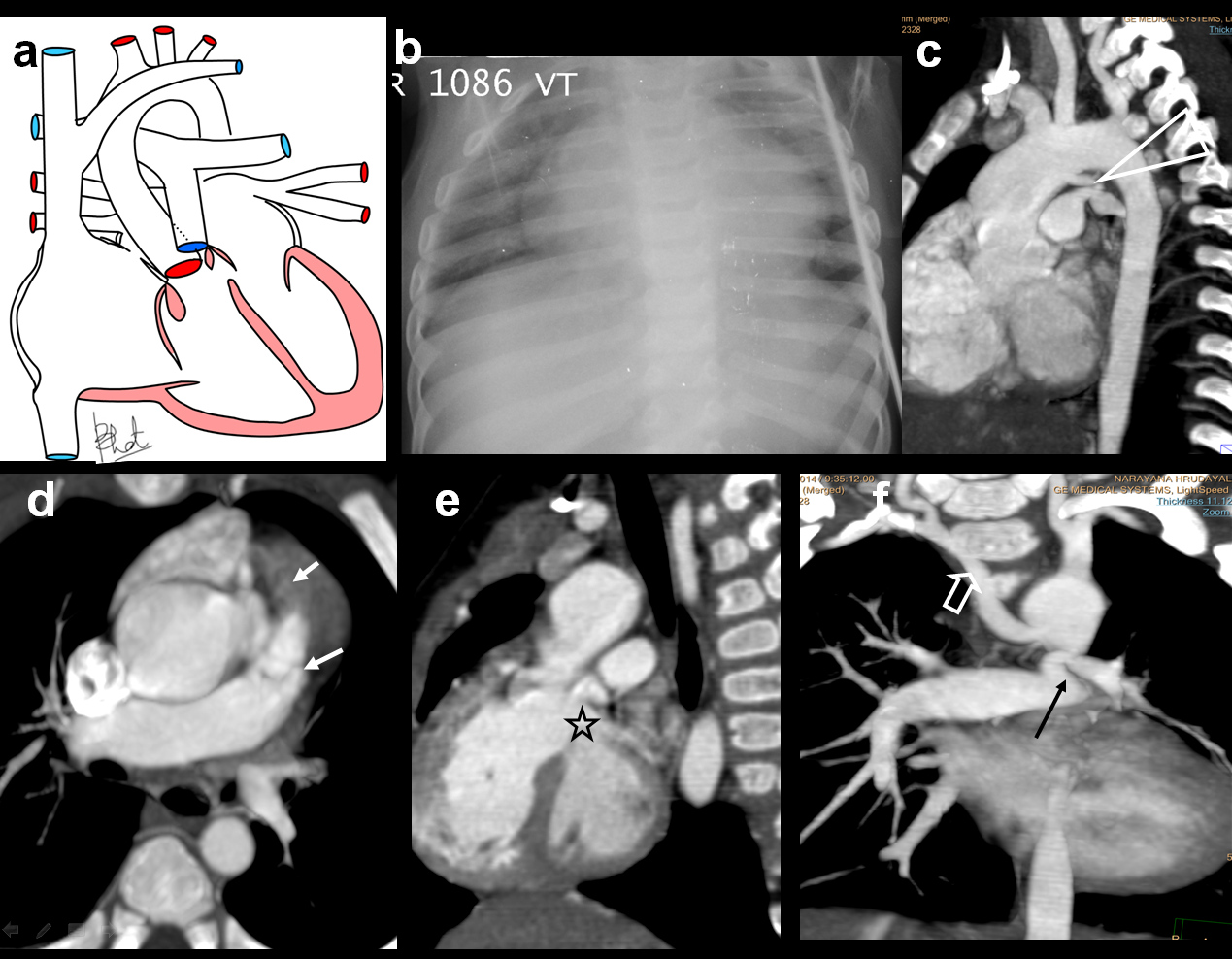
Double outlet right ventricle (DORV) with aberrant right subclavian artery.
One-year-old male referred to exclude CHD, diagnosed with a DORV in association with ARSA; (a) Diagrammatic representation of vascular anatomy in DORV, both great arteries arising from RV; (b) Plain radiograph reveal cardiomegaly with oligemic lungs; (c) Sagittal oblique MIP views shows dilated aortic arch with normal branching. There is a small PDA(triangle); (d)Axial MIP images shows infundibular and valvular stenosis(arrows); (e) Outflow view shows both aorta and PA arising from RV, LV communicating with a large VSD(star); (f) CT Coronal MIP view shows ARSA (open arrow) and narrowing of origin of LPA (arrow).
DORV, anomalons conus branch.
One-year-old male presented with the cyanosis on feeding and feeding difficulty, suspected as TOF on echocardiography , diagnosed with DORV: (a) Diagrammatic representation patient of vascular anatomy in DORV; (b) Plain radiograph shows right ventricular cardiac configuration, oligemic lungs and right aortic arch (arrow); (c) Outflow view shows both aorta and PA arising from RV, LV communicating with a large VSD(arrow); (d) Axial CTA reveals normal size pulmonary arteries and right aortic arch (arrow) (e) Axial CT at aortic root show conus branch crossing RTOT(open arrow); (f) Coronal CT reconstruction shows tight infundibular stenosis (open arrow).
Transposition of Great arteries (TGA) [Table/Fig-14a&b]: Transposition of great artery is the most common cyanotic heart disease in the neonatal group. Depending on chamber-vessel connection there are two entities; D-TGA and LTGA. In D TGA, there is ventricular-arterial discordance with anterior location of the aorta, arising from the right ventricle [Table/Fig-15a&b]. Coronary arteries arise from aorta, from posterior cusps. Due to atrio-ventricular concordance and ventriculo-arterial discordance, systemic venous blood passes through the right heart to the aorta and pulmonary venous blood passes through the left heart to the lungs. Survival is dependent on the existence of a communication between pulmonary and systemic circulations (patent ductus arteriosus, ventricular septal defect, or atrial septal defect) [17]. Plain radiography in appropriate clinical setting, often provide clue to the diagnosis. Mild biventricular cardiomegaly (egg on side) with a narrow cardiac pedicle in a cyanotic neonate suggests the diagnosis of transposition of great arteries. Lungs in most case show increased arterial flow pattern. The immediate postnatal palliative procedure required in patients with D-TGA is to create an atrial septal defect. Total repair of D-TGA involves performing the rerouting the systemic return to left atrium through a channel (Senning procedure) and directing pulmonary return to the right atrium. In Rastelli procedure transventricular rerouting of the left ventricular outflow is performed. Pulmonary trunk is divided and a conduit is placed within right ventricle and the pulmonary trunk. Jantane procedure is a 3-stage arterial switch procedure. Imaging by MDCT and /or MRI is helpful in demonstrating a post-procedural complications like stenosis at various sites of anastomosis [4,9]. In L-TGA there is a discordant atrio-ventricular and ventricular arterial connection. Some patients may be asymptomatic or present late. Treatment involves the double switch procedure (Senning of mustard) or Rastelli’s repair [9].
| Subtypes | Complete TGA (D-TGA) | Congenitally corrected TGA (L-TGA) |
|---|
| D-TGA (Dextro-TGA)= aorta anterior and to the right of pulmonary artery, Complete separation of systemic and pulmonary circulations AV concordance and VA discordance (Aorta arising from RV and PA arising from LV) |
| Incidence [13] | 1 in 4000 live births. M: F=2:1 to 3:1. Increased risk in infants of diabetic mothers. |
| Association | VSD/ASD/PFO= necessary for survival LVOT obstruction (dynamic or fixed) coexisting with VSD Pulmonary valve stenosis. |
| Syndromes | Laurence-Moon-Biedl syndrome. |
| Imaging features Plain | “Egg on a string” sign combination of abnormal great vessel anatomy + apparent narrowing of superior mediastinum by hyperinflated lungs and stress-induced thymic atrophy |
| Imaging features specific | Echo Direct demonstration MRI useful for post-surgical follow-up for assessment of degree of systemic RV failure and tricuspid regurgitation, to look for intraatrial baffle leak or obstruction and to rule out obstruction of systemic/pulmonary venous pathways CT |
| Management [13] | Palliative:1. Rashkind procedure: balloon atrial septostomyDefinitive repair:
Atrial baffle operations: Mustard and Senning Arterial switch surgery (Jatene)- procedure of choice Rastelli operation: Intraventricular baffle to close VSD and redirect L outflow tract to aorta (useful in presence of both VSD and pulmonary stenosis)
|
| L-TGA (Levo-TGA)= aorta anterior and to the left of pulmonary artery AV and VA discordance (Aorta arising from right sided LV and PA arising from left sided RV) |
| Incidence [13] | 1 in 13,000 live births. M>F. |
| Association | VSD, pulmonary stenosis (valvular or subvalvular), abnormalities of systemic AV (tricuspid) valve and conduction system anomalies |
| Syndromes | Laurence-Moon-Biedl syndrome |
| Imaging features Plain | Cardiomegaly with straight left upper heart border | Pulmonary Plethora (in TGA + VSD) | LA enlargement and pulmonary venous congestion (TGA + severe left AV valve regurgitation) |
| Imaging features specific | Echo Direct demonstration MRI and CT for demonstration of cardiac/extracardiac anomalies |
| Management [12] | Palliative:
Modified BT shunt (in pts with severe PS) Pulmonary artery banding for uncontrollable CHF in infancy
Definitive repair:
Classic repair: leaves the anatomical RV as systemic ventricle Anatomic repair: makes the anatomical LV as systemic ventricle; less risk of post-op TR and RV failure
|
TGA. A two-month-old male cyanotic child, suspected on echocardiography to have TGA, large VSD PS, diagnosed with transposition of great arteries, VSD and PS: (a) diagrammatic illustration of TGA with narrowing of the pulmonary outflow; (b) Plain radiography demonstrate mild biventricular cardiac enlargement. Lung fields are unremarkable; (c) Axial MIP image showing anteriorly located large aorta (arrow) and posteriorly located pulmonary artery (and open arrows); (d) CT axial image demonstrates biventricular enlargement with large ventricular septal defect (circle).
A two-month-old male cyanotic child, suspected on echocardiography to have TGA, large VSD PS, diagnosed with transposition of great arteries, VSD and PS; (e) Lateral MIP CT image demonstrates anterior location of aorta (arrow). There is a narrowing of the pulmonary arterial origin (open arrow) (f). Modified oblique view shows large ventricular septal defect, anteriorly located aorta (arrow) and posterior pulmonary artery with narrowing at the origin. (triangle).
Truncus arteriosus (TA) [Table/Fig-16]: Truncus arteriosus is an uncommon cono-truncal anomaly characterized by a single arterial vessel that originates from the heart, overrides the ventricular septum, and supplies the all the three, systemic, pulmonary, and coronary circulation. There is well known association with DiGeorge syndrome and chromosome 22q11 deletion [20]. The classification of truncus arteriosus is made on branching pattern of the pulmonary artery, described originally by Collett and Edwards [21] modified subsequently by Van Praagh [22]. Echocardiography is adequate for diagnosis and surgical planning in most of patients. MRI or MDCT may be needed for delineation of branching pattern of pulmonary arteries, demonstration of aortopulmonary collateral vessels, pulmonary venous abnormalities and configuration of aortic arch [Table/Fig-17a&b]. MR imaging is most optimal in evaluating in the assessment of postoperative complications [17].
| Subtypes: Collett and Edwards classification [18] | 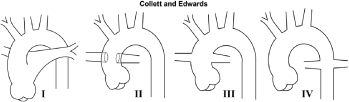 Type I = pulmonary trunk arises from proximal truncusType II and III= no pulmonary trunk; branch PA arise from posterior and lateral mid-segments of truncusType IV=pulmonary circulation dependent on MAPCAs. Type I = pulmonary trunk arises from proximal truncusType II and III= no pulmonary trunk; branch PA arise from posterior and lateral mid-segments of truncusType IV=pulmonary circulation dependent on MAPCAs. |
| Incidence | 1% of CHD. 1 in 11,000 live births(10–5) |
| Association [13] | Right aortic arch, interrupted aortic arch, coarctation, PDA Coronary artery anomalies(stenotic coronary ostia, abnormal branching and course) Unilateral absence of pulmonary artery |
| Syndromes | DiGeorge syndrome Pierre Robin syndrome |
| Imaging features Plain | Moderate cardiomegaly with narrow base | High cephalic origin of PA | Right aortic arch | Depressed diaphragm | Thymic atrophy |
| Imaging features specific | Echo large truncus, large VSD and a large echogenic truncal valve with fibrous continuity with anterior mitral leaflet MRI -similar findings |
| Management | Primary repair: done in first week of life; consists of VSD closure and placement of valve conduit between RV and PA (Rastelli procedure) |
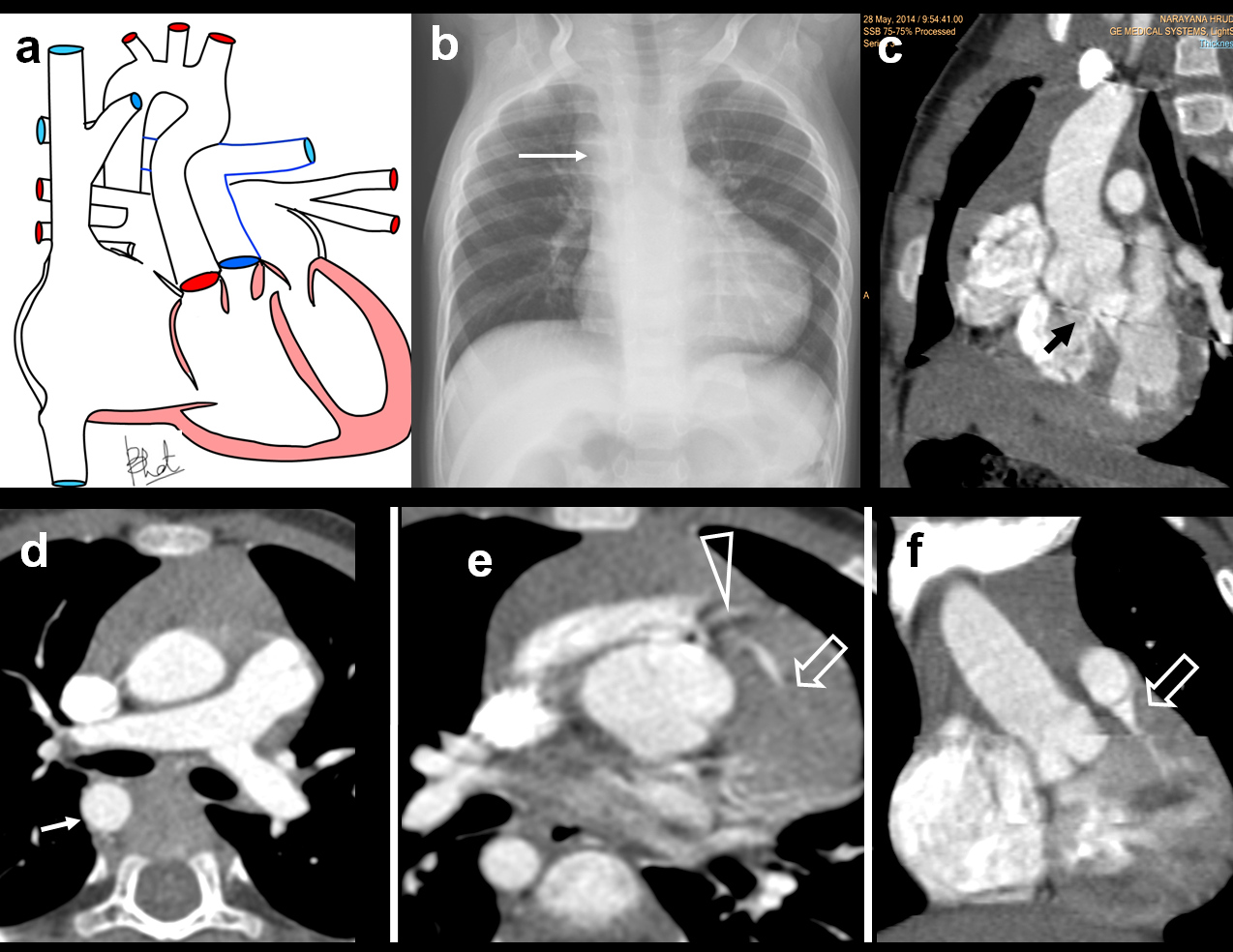
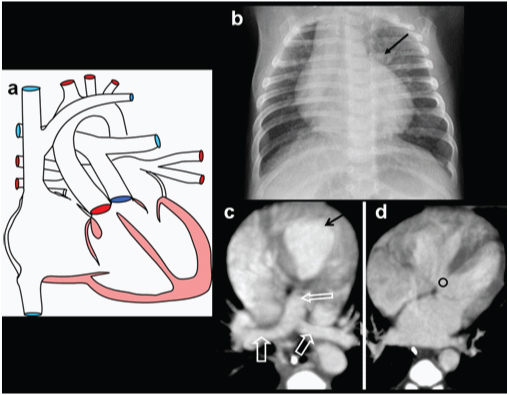
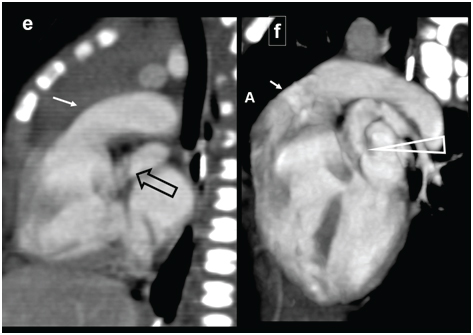
Truncus arteriosus. A three-month-old male with breathing difficulty, recurrent chest infection, diagnosed with truncus arteriosus: (a) Diagrammatic representation of vascular anatomy in truncus arteriosus; (b) Plain radiograph shows moderate cardiac enlargement with increased pulmonary arterial markings, especially on right side(open arrow); (c) echocardiography shows a single trunk(open arrow) providing origin to pulmonary artery and aorta; (d-f) Reconstructed outflow CT images shows a single large ascending aorta overriding large septal defect(circle); (e) Reconstructed axial oblique CT view shows pulmonary arteries arising from posterior aspect of aorta (triangle).
A three -month-old male with breathing difficulty, recurrent chest infection, diagnosed with truncus arteriosus; (a) Axial CT view shows configuration of aortic arch (arrow) and origin of pulmonary artery(arrow); (b) 3 D reconstruction showing larger aorta (arrow) and RPA and LPA arising posteriorly (open arrows). Also, there is an aberrant left subclavian artery (triangle).
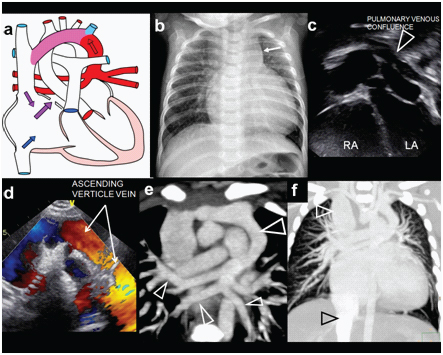
Total anomalous pulmonary venous connection (TAPVC):
Supracardiac [Table/Fig-18] Intracardiac [Table/Fig-19] and infracardaic types [Table/Fig-20]. These anomalies involve drainage of the pulmonary veins to the systemic circulation. Intracardiac TAPVC is associated with atrial isomerism (polysplenia or asplenia). Anomalous pulmonary venous drainage can be associated with obstruction, especially infracardiac variety. Plain radiography characteristically shows signs of severe pulmonary oedema or congestion with normal cardiac size in obstructed TAPVC [23]. In supracardiac TAPVC shows cardiomegaly with wide mediastinum and plethoric lungs. (Snow man or Figure of ‘8’ heart) Imaging by CT or MRI plays a greater role in imaging these anomalies [Table/Fig-21a&b]. Even non-gated MDCT examinations provide accurate delineation of Anatomy, much superior to trans-esophageal echography [24] Prompt surgical relief of obstruction is required on an urgent basis [9]. Corrective procedure involves the reconnection of the pulmonary vein to the left ventricle. Prognosis is unfavorable for infra diaphragmatic TAPVC. Examples of intracardiac [Table/Fig-22a&b] and Infracardiac TAPVC [Table/Fig-23a,b] are illustrated in the images.
| Subtypes | supracardiac | cardiac | infracardiac | mixed |
|---|
| SupracardiacTapvc | Pathway of pulmonary venous return:Common pulmonary vein left vertical vein left innominate vein right SVC right atrium |
| Incidence [13] | Accounts for 50% of TAPVC patients. |
| Association | ASD/PFO Heterotaxy syndrome |
| Syndromes | |
| Imaging features Plain | “snowman sign or figure-of-8 configuration” = dilated vertical vein on the left, the innominate vein on the top, and the superior vena cava on the right form the head of the snowman; the body of the snowman is formed by the enlarged right atrium. | Pulmonary plethora |
| Imaging features specific | Echo demonstrates the anomalous pulmonary venous drainage MRI best for hemodynamic assessment CT |
| Management [8,9,13] | Surgical rerouting- by large side-to-side anastomosis between common pulmonary venous pathway and LA with ligation of vertical vein and closure of ASD |
| cardiac tapvc | Pathway of pulmonary venous return:
Common pulmonary venous channel coronary sinus RA. All 4 pulmonary veins drain separately into RA.
|
| Incidence [13] | Accounts for 20% of TAPVC patients. |
| Association | ASD/PFO |
| Syndromes | |
| Imaging features Plain | Moderate cardiomegaly (RA/RV enlargement) | Pulmonary plethora |
| Imaging features specific | Echo dilated coronary sinus- first clue to this conditionMRI adequately demonstrates the anomalous pathway with functional assessment (Qp:Qs) CT |
| Management [13] | TAPVC to RA: excision of atrial septum and redirection of pulmonary venous return to LA through a patch TAPVC to coronary sinus: Unroofing of coronary sinus (incision in anterior wall of coronary sinus for communication between CS and LA) with closure of ostium of CS and ASD
|
| Infracardiac Tapvc | Pathway of pulmonary venous return: Common pulmonary venous channel drains into portal vein, ductus venosus, hepatic vein or IVC by crossing the diaphragm through esophageal hiatus |
| Incidence [13] | Accounts for 20% of TAPVC patients. Marked male preponderance with M:F ratio of 4:1. Most of infracardiac TAPVC are obstructed. Hence it is considered as one of the paediatric cardiac emergency. |
| Association | ASD/PFO |
| Syndromes | |
| Imaging features Plain | Normal or mild cardiomegaly | Pulmonary oedema features (diffuse reticular pattern and Kerley B lines) |
| Imaging features specific | Echo - dilated descending vein passing through diaphragm and draining into systemic veins. MRI best suited for post-op follow-upCT useful in features of obstruction on ECHO with unclear site of obstruction |
| Management [13] | Surgical: Large vertical anastomosis between common pulmonary venous channel and LA with ligation of common channel above the diaphragm |
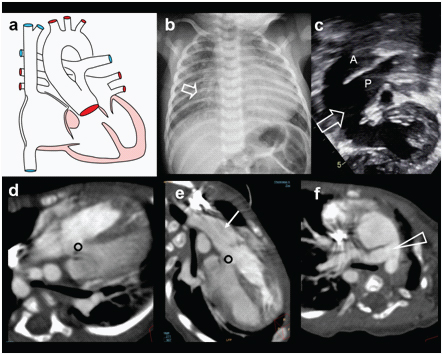
Supracardiac TAPVC. A three-month-old female presenting with cyanosis while feeding, abnormal vessel on echography, diagnosed with TAPVC: (a) diagrammatic representation of confluence of pulmonary veins into a vertical vein, draining subsequently in to left innominate vein; (b) Plain radiography demonstrating biventricular cardiomegaly and plethoric lung fields. Widening of the mediastinum is noted on left side (arrow); (c,d) Grey scale and colour Doppler echocardiography demonstrate ascending vertical vein (triangle and arrows) Both atria are enlarged; (e) MIP image in coronal plane of contrast-enhanced CT demonstrates confluence of all pulmonary veins into vertical vein and innominate vein. (Triangles); (f) Coronal CT MIP image demonstrates pulmonary plethora, dilated MPA and vena-cavae (triangles).
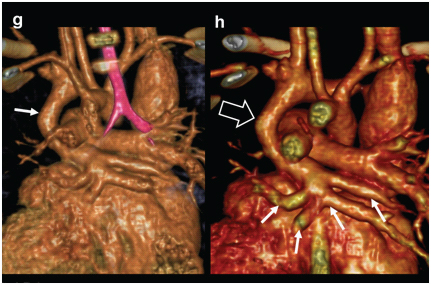
Supracardiac TAPVC. A three-month-old female presenting with cyanosis while feeding, abnormal vessel on echography, diagnosed with TAPVC (g, h) 3-D CT reconstructed images viewed from posterior aspect demonstrates anomalous drainage of all the pulmonary veins into vertical vein (arrow).
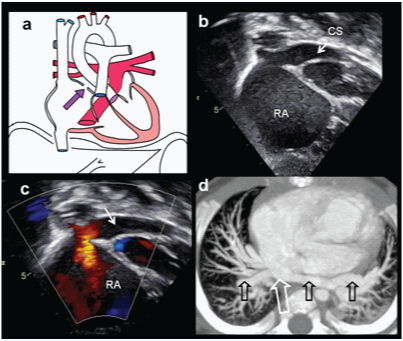
Intra-cardiac TAPVC. A two-month-old female presenting with cyanosis while crying, shown to have a septal defect on echocardiography diagnosed with intra-cardiac total anomalous pulmonary venous drainage: (a) Diagrammatic illustration showing a confluence of pulmonary artery draining into the enlarged coronary sinus; (b) Echocardiography shows dilated coronary sinus draining in to enlarged right atrium (arrow); (c) Colour Doppler exam shows enhanced flow in to right atrium via coronary sinus; (d) Axial CT MIP image shows confluence of pulmonary veins and dilated coronary sinus, draining into right atrium (open arrows).
A two-month-old female presenting with cyanosis while crying, shown to have a septal defect on echocardiography diagnosed with intra-cardiac total anomalous pulmonary venous drainage: (a) axial CT images the level of pulmonary artery revealed grossly dilated MPA (open arrow); (b) axial CT image demonstrates grossly enlarged right atrium with a large secundum septal defect (open arrow). There is enlargement of the both ventricular cavities; (c) Coronal CT MIP image shows confluence of pulmonary veins (arrows); and (d) increased pulmonary arterial vascularity.
Infra-cardiac TAPVC. 3-day-old female neonate presenting with respiratory distress, diagnosed with infra-diaphragmatic TAPVC to portal vein on MDCT. (a) Diagrammatic representation of vascular anatomy in infra-cardiac TAPVC. Anomalous common pulmonary vein (CPV) is shown draining to portal vein (colored red); (b) Plain radiograph shows obscured cardiac shadow with evidence of pulmonary oedema; (c)Coronal MIP CT reconstruction show anomalous CPV traversing the diaphragm and joining portal vein (arrow). Minimal narrowing noted at diaphragmatic level (triangle); (d,e) Axial CT images shows confluence of pulmonary veins (star) and site of union with portal vein (open arrow).
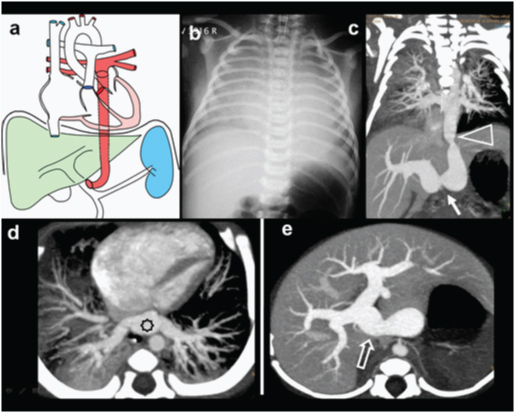
A three-day-old female neonate presenting with respiratory distress, diagnosed with infra-diaphragmatic TAPVC to portal vein on MDCT: (a) 3 D rendered CT image viewed from posterior aspect shows the course of common pulmonary vein (arrows); (b) Sagittal MIP CT reconstruction show anomalous common pulmonary vein traversing the diaphragm with minimal narrowing at diaphragmatic level (triangle).
Ebstein’s Anomaly [Table/Fig-24]: On plain radiography Ebstein’s anomaly has the classic appearance of ‘box shaped heart’. Large heart size is common in Ebstein’s anomaly due to massively enlarged right atrium, which can also cause a posterior bulge in lateral chest radiograph [25]. Adequate evaluation can be performed by echocardiography and MR imaging [Table/Fig-25]. Typically there is caudal displacement, dysplasia of septal and posterior leaflets of tricuspid valve, dilatation of the right atrium and atrialized portion of the RV, which may pulsate paradoxically in ventricular systole [9]. Associated cardiac anomalies and conduction defects can be observed. MR imaging is superior in demonstrating morphological features, chamber contractility and measuring regurgitant tricuspid flow, which has prognostic value [4] Management of Ebstein’s anomaly depends upon the age of patient, severity of the malformation, degree of right ventricular outflow tract obstruction and dynamic status of pulmonary vascular resistance. The surgical approach depending on the exact context involve ligation of a patent ductus arteriosus, placement of a systemic to pulmonary shunt, tricuspid valve repair or reducing flow across tricuspid valve [26].
| Ebsteins anomaly of tricuspid valve [25,27] | Characterized by downward displacement of septal and posterior leaflets of tricuspid valve into RV cavity resulting in atrialisation of a portion of RV with smallish functional RV. |
| Subtypes: Carpenter’s classification |
| Incidence [13,27] | <1% of all CHDs. |
| Association | Maternal lithium intake especially in first 8 weeks of gestation is highly associated with this anomaly.ASD with right-to-left shunt |
| Syndromes |
| Imaging features Plain | “Box shaped heart” = secondary to hugely dilated RA which may fill the entire right hemithorax, normal sized LA with shelved appearance of left cardiac contour (due to dilated RVOT), small aorta, discrete convex bulge of MPA | Pulmonary oligemia |
| Imaging features specific | Echo apical displacement of hinge point of septal leaflet of tricuspid valve (Criteria for diagnosis: apical displacement by > 8 mm/mm2of BSA); huge RA; small functional RV with dilated RVOT MRI useful in pre- and post-surgical patients CT |
| Management | Surgical repair |
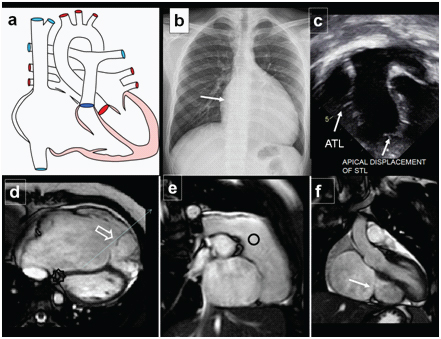
Ebsteins Anomaly. A 17-year-old male presenting with cyanosis on diagnosed with Ebsteins anomaly on Echocardiography and MR: (a) Diagrammatic representation of cardiac anatomy in Ebsteins anomaly; (b) Plain radiograph shows enlarged cardiac shadow, RA enlargement (arrow) and pulmonary oligmia; (c) Echocardiography demonstrating enlarged RA with apical displacement of septal tricuspid leaflet (arrow); (d) Axial bright blood MRI examination shows dilated RA displaced dysplastic tricuspid valves (open arrow); (e) Sagittal images shows large RA cavity and RV outflow tract(circle); (f) Coronal images illustrates displaced tricuspid valve (arrow).
There are few additional cardiac anomalies which have not been illustrated in the presentation. Notable entities include a tricuspid atresia, Cor tri-atriatum, severe isolated valvular atresia and hypoplastic ventricles. Evaluation of these entities is done with the same guidelines utilising combination of echocardiography, plain radiography, MRI, CT and cardiac catheterization. Specific imaging appearances are well described [9,13,28].
Conclusion
In this concluding session on imaging of CHD, an approach to the management of cyanotic and complex congenital heart disease is provided. Multimodality approach with use of more than one imaging modality, including invasive intravascular studies are often required in order to plan and explore the potential of available treatment options. Advances in surgical options, longer survival of patients on palliation have triggered advanced techniques in MR and CT imaging. This presentation highlights imaging appearances of commonly occurring cyanotic and complex CHD like Tetralogy and variants, TGA and other less common complex conditions in the form of illustrated case studies.
[1]. Bhat V, Belaval V, Karthik GA, Illustrated Imaging essay on Congenital Heart Diseases: Multimodality approach Part I: Clinical context, anatomy and Imaging techniquesJ Clin Diagn Res 2016 10(5):TE01-06. [Google Scholar]
[2]. Bhat V, Belaval V, Karthik GA, Illustrated Imaging essay on Congenital Heart Diseases: Multimodality approach Part II: Acyanotic congenital heart disease and Extracardaic abnormalitiesJ Clin Diagn Res 2016 10(6):TE01-06. [Google Scholar]
[3]. Bailliard F, Hughes ML, Taylor AM, Introduction to cardiac imaging in infants and children: techniques, potential, and role in the imaging work-up of various cardiac malformations and other paediatric heart conditionsEur J Radiol 2008 68(2):191-98. [Google Scholar]
[4]. Kilner PJ, Imaging congenital heart disease in adultsBr J Radiol 2011 84(Spec No 3):S258-68. [Google Scholar]
[5]. Goo HW, Cardiac MDCT in children: CT technology overview and interpretationRadiolClin North Am 2011 49(5):997-1010. [Google Scholar]
[6]. Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Buechel ERV, Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart diseaseJ Cardiovasc Magn Reson 2013 15(1):51 [Google Scholar]
[7]. Ntsinjana HN, Hughes ML, Taylor AM, The role of cardiovascular magnetic resonance in paediatric congenital heart diseaseJ Cardiovasc Magn Reson 2011 13(1):51 [Google Scholar]
[8]. Kumar A, Patton DJ, Friedrich MG, The emerging clinical role of cardiovascular magnetic resonance imagingCan J Cardiol 2010 26(6):313-22. [Google Scholar]
[9]. Singh SP, Congenital heart diseaseIn: Specialty Board Review RADIOLOGY, Cheri L. Canon, (ed)New York:289-317.Copyright © 2010 [Google Scholar]
[10]. Raman SV, Cook SC, McCarthy B, Ferketich AK, Usefulness of multidetector row computed tomography to quantify right ventricular size and function in adults with either tetralogy of Fallot or transposition of the great arteriesAm J Cardiol 2005 95(5):683-86. [Google Scholar]
[11]. Gulati GS, Singh C, Kothari SS, Sharma S, An unusual coronary artery anomaly in tetralogy of Fallot shown on MDCTAJR Am J Roentgenol 2006 186(4):1192-93. [Google Scholar]
[12]. Rajeshkannan R, Moorthy S, Sreekumar KP, Ramachandran PV, Kumar RK, Remadevi KS, Role of 64-MDCT in evaluation of pulmonary atresia with ventricular septal defectAJR Am J Roentgenol 2010 194(1):110-18. [Google Scholar]
[13]. Myung K, Park; Paediatric Cardiology for Practitioners 2007 5th editionMosby, Elsevier HealthPt IV, Ch 16, pp 174-240 [Google Scholar]
[14]. Rabinovitch M, Grady S, David I, Compression of intrapulmonary bronchi by abnormally branching pulmonary arteries associated with absent pulmonary valvesAm J Cardiol 1982 50(4):804-13. [Google Scholar]
[15]. Galindo A, Gutierrez-Larraya F, Martinez JM, Prenatal diagnosis and outcome for fetuses with congenital absence of the pulmonary valveUltrasound Obstet Gynecol 2006 28(1):32-39. [Google Scholar]
[16]. Chelliah A, Berger JT, Blask A, Donofrio MT, Clinical utility of fetal magnetic resonance imaging in tetralogy of Fallot with absent pulmonary valveCirculation 2013 127(6):757-59. [Google Scholar]
[17]. Frank L, Dillman JR, Parish V, Mueller GC, Kazerooni EA, Bell A, Cardiovascular MR imaging of conotruncal anomaliesRadiographics 2010 30(4):1069-94. [Google Scholar]
[18]. Cetta F, Boston US, Dearani JA, Hagler DJ, Double outlet right ventricle: opinions regarding managementCurr Treat Options Cardiovasc Med 2005 7(5):385-90. [Google Scholar]
[19]. Chen SJ, Lin MT, Liu KL, Chang CI, Chen HY, Wang JK, Usefulness of 3D reconstructed computed tomography imaging for double outlet right ventricleJ Formos Med Assoc 2008 107(5):371-80. [Google Scholar]
[20]. Goldmuntz E, Clark BJ, Mitchell LE, Frequency of 22q11 deletions in patients with cono-truncal defectsJ Am Coll Cardiol 1998 32(2):492-98. [Google Scholar]
[21]. Collett RW, Edwards JE, Persistent truncus arteriosus:a classification according to anatomic typesSurg Clin North Am 1949 29:1245-70. [Google Scholar]
[22]. Van Praagh R, Van Praagh S, The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications: a study of 57 necropsy casesAm J Cardiol 1965 16(3):406-25. [Google Scholar]
[23]. Shen Q, Pa M, Hu X, Wang J, Role of plain radiography and CT angiography in the evaluation of obstructed total anomalous pulmonary venous connectionPaediatr Radiol 2013 43(7):827-35. [Google Scholar]
[24]. Yao Q, Hu X, Pa M, Huang G, Non-ECG-gated MDCTA of infracardiac total anomalous pulmonary venous connection in neonates and young infantsHerz 2013 38(5):539-43. [Google Scholar]
[25]. Deutsch V, Wexler L, Blieden LC, Yahini JH, Neufeld HN, Ebstein’s anomaly of tricuspid valve: critical review of roentgenological features and additional angiographic signsAm J Roentgenol Radium Ther Nucl Med 1975 125(2):395-411. [Google Scholar]
[26]. Jaquiss RD, Imamura M, Management of Ebstein’s anomaly and pure tricuspid insufficiency in the neonateSemin Thorac Cardiovasc Surg 2007 19(3):258-63. [Google Scholar]
[27]. Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK, Ebstein’s anomaly - review of a multifaceted congenital cardiac conditionSwiss Med Wkly 2005 135(19-20):269-81. [Google Scholar]
[28]. Elagha AA, Fuisz AR, Weissman G, Cardiac magnetic resonance imaging can clearly depict the morphology and determine the significance of cortriatriatumCirculation 2012 126(12):1511-13. [Google Scholar]