Dental units are the core of dental clinics. The air–water syringes, ultrasonic scalers, prophy-angles, high speed air turbine handpieces are connected to dental units by a network of small-bore plastic tubes through which water and air travel to activate or cool the instruments. Various authors have shown that Dental Unit Waterlines are extensively contaminated with microbial biofilms [1]. Microorganisms of atleast 40 different species have been identified including: oral streptococci, Pseudomonas spp., Enterobacteria, Candida albicans, Legionella pneumophila and non– tuberculous Mycobacterium spp. Planktonic forms of microorganisms and pieces of biofilm are shed from the Dental Unit Waterlines (DUWLs) and are then transferred directly into the mouths of patients during dental procedures and represent a potential source of infection for both patients as well as Dental Health Care Personals (DHCPs) [1]. Direct sources of DUWL contamination are municipal water piped into dental units, independent reservoirs and suck back patient’s saliva [2–4]. An indirect source of contamination within the waterlines is a biofilm developing in small-bore plastic tubing [2,5].
The problem of DUWL contamination has been recognized for many years. The American Dental association (ADA) set a goal to reduce the number of noncoliform, mesophilic, heterotrophic bacteria in patient treatment water to 200 CFU/ml by the year 2000 [6]. The Centers for Disease Control and Prevention (CDC) in 2003 published “Guidelines for Infection Control in Dental Health-care Settings” and recommended ≤500 CFU/mL for nonsurgical dental procedures [7]. For surgical procedures sterile irrigant water or saline provided from a separate and preferably single use source should be used [8].
Flushing or purging the waterlines had been recommended to improve the quality of dental water [9,10]. Physical decontamination can be done by using synthetic membranes for the filtration of water and drying. Chemical decontamination using different disinfectants like peracetic acid, hydrogen peroxide, silver salts, chloramine, gluteraldehyde, chlorhexidine, chlorine dioxide, EDTA and sodium hypochlorite have been evaluated by various authors [1,3,5]. Newer methods include ozonation of water, anti-retraction devices in dental turbines and auto flushing dental units. Out of all the methods of prevention of cross contamination of DUWL’s, chemical disinfection is well accepted, practical, cost-effective and evidence based method [10].
Despite of this fact, India still lacks availability of such disinfectants and those available are priced very high. So, the current study was conducted to evaluate and compare efficacy of various disinfectants which are very easily available and cost-effective, in reducing the microbial colony count in water derived from DUWLs through high speed hand piece and air-water syringe.
Materials and Methods
This experimental study was conducted to evaluate and compare efficacy of various disinfectants in reducing the microbial colony count in water derived from DUWLs through high speed hand piece and air-water syringe at Department of Prosthodontics, Maharishi Markandeshwar College of Dental Sciences & Research, Mullana, Ambala, Haryana, India. Five Freshly prepared disinfectants with various disinfection protocols were used for disinfection of dental unit waterlines for a period of 4 weeks. Before starting the disinfection regime samples were collected from all the labeled units for baseline counts.
Disinfectant concentrations were prepared from commercially available laboratory reagents and distilled water.
Five random dental units with independent reservoirs were selected and were labeled as group A, B, C, D and E according to the type of disinfectant used and the method of disinfection.
Group A: 0.02% H2O2 continuously: 0.02% H2O2 solution (prepared by dissolving 3.33 ml of 6% H2O2 in 1 L of distilled water) (Hydrogen Peroxide solution 6% w/v, Thermo Fisher Scientific India Pvt. Ltd.) was added in the independent reservoir of dental unit and was used continuously.
Group B: 0.02% H2O2 continuously with shock treatment with 0.25% H2O2 every week: 0.02% H2O2 solution (prepared by dissolving 3.33 ml of 6% H2O2 in 1 L of distilled water) solution was added in the independent reservoir of dental unit and was used continuously. An additional shock treatment with 0.25% H2O2 (prepared by dissolving 41.6 ml of 6% H2O2 in 1 L of distilled water) was done every weekend. It included circulation of 0.25% H2O2 for 5 minutes every Saturday at the end of clinical hours. The solution was left in DUWL for entire weekend. The dental unit was flushed for 5 minutes on Monday morning and was again replaced by 0.02% H2O2 as continuous disinfectant.
Group C: 0.12% Chlorohexidine and 12% Ethanol overnight: 0.12% Chlorohexidine (prepared by dissolving 6 ml of 20% chlorohexidine gluconate in distilled water) (Chlorohexidine Gluconate pure 20%, Otto Chemie Pvt Ltd, India.) and 12% ethanol (prepared by dissolving 120 ml of absolute alcohol in 1 L of distilled water) (Ethanol absolute, Changshu Yangyuan Chemical, China) was added in independent reservoir at the end of the working time. The DUWL was flushed with the solution for 2 minutes so that solution reached all the tubes till the exit points and was allowed to stand for overnight. After that the solution was replaced with distilled water (Distilled Water, Swastika Bio Remedies Pvt. Ltd, India.) and dental unit was again flushed for 2 minutes so that all the disinfectant was washed away from the tubings before its use.
Group D: 1:50 Original Listerine overnight: Overnight treatment with 1:50 Original Listerine (prepared by dissolving 20 ml of Original Listerine in 1 L distilled water) (Listerine, Johnson & Johnson Ltd, India.) was done in a similar way as in group C. Group E: 2% Sodium Perborate and 2% EDTA 5 minutes in morning: Disinfectant solution consisting of 2% Sodium Perborate (prepared by dissolving 20 mg of Sodium Perborate in 1 L of distilled water) (Sodium Perborate tetrahydrate, Central Drug House Pvt Ltd, India.) and 2% EDTA (prepared by dissolving 20 mg each of EDTA in 1 L of distilled water) (Ethylenediamine tetraacetic acid Extra Pure, Merck specialties Pvt. Ltd, India.) was added every morning in the independent reservoir and the waterlines were flushed for 2 minutes so that disinfectant solution was introduced in all the tubings. The solution was allowed to stand for 5 minutes. After that solution was replaced by distilled water and dental unit was again flushed for at least 2 minutes to remove all the disinfectant solution from the waterlines.
Before collection of samples all dental treatment water outlets were flushed for 30 seconds followed by cleaning with a spirit pad. Then from each individual unit, pooled water samples were collected into a 20 milliliter sterile collection bottle containing sodium thiosulphate (to eliminate residual disinfectant) from two outlets (high-speed hand-piece water line and 2- way air-water syringes). Five samples were collected from each dental unit each time (baseline sample on Monday morning before disinfection, one day after disinfection and weekly for consecutive 4 Monday mornings) and were labeled. A total of 150 samples were collected during the study [Table/Fig-1].
| Groups | IBaseline | II1 day | III1 week | IV2 weeks | V3 weeks | VI4 weeks | Total |
|---|
| Group A0.02% H2O2continuously | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Group B0.02% H2O2 continuously with shock treatment with 0.25% H2O2 | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Group C0.12% CHX & 12% ethanol – overnight | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Group D1:50 original Listerine – overnight | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Group E2% Sodium Perborate + 2% EDTA5 mins in morning | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
| Total number of Samples | 150 |
The water samples collected at each time interval were transported immediately to Department of Microbiology, Maharishi Markandeshwar College of Medical sciences & Research, Mullana, Ambala. The samples were serially diluted (dilution factor -100) with sterile water (Sterile Water For Injection, Zydus Cadila Healthcare Ltd, India.) and 1ml of each sample was cultured on R2A agar (R2A Agar, Titan Biotech Pvt Ltd, India.) plate with spread plate method. All samples were incubated at 22oC-28oC for 7 days. After 7 days the cultured plates were taken out and the colony forming units were counted manually using microbial colony counter (Spectronics, Panchkula, India). The results were recorded for each cultured water samples for colony forming units per ml (cfu/ml) and were evaluated statistically using one-way ANOVA [Table/Fig-2] and paired t-test [Table/Fig-3].
Mean values of CFU/ml after the microbiological culture of the DUWL samples and oneway-ANOVA.
| Groups | Subgroups | One-way ANOVA |
|---|
| IBaseline | II1 day | III1 week | IV2 weeks | V3 weeks | VI4 weeks | F | Sig. |
|---|
| Group A 0.02% H2O2 continuously | 1240000 | 40000 | 9000 | 1100 | 200 | 200 | .120 | 0.974* |
| Group B 0.02% H2O2 continuously with shock treatment with 0.25% H2O2 | 1120000 | 20000 | 4000 | 3500 | 3000 | 700 | .119 | .975* |
| Group C 0.12% CHX & 12% ethanol - overnight | 1520000 | 940000 | 23000 | 7300 | 4000 | 100 | .176 | 0.949* |
| Group D 1:50 original Listerine - overnight | 1440000 | 120000 | 20000 | 3300 | 1100 | 200 | .207 | 0.932* |
| Group E 2% Sodium Perborate + 2% EDTA 5 mins in morning | 1260000 | 390000 | 13000 | 7400 | 1500 | 300 | .046 | 0.996* |
*Non Significant
Paired Samples Test for groups.*
| Paired Differences | t | df. | Sig. (2-tailed) | Remarks |
|---|
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference |
|---|
| Lower | Upper |
|---|
| Group A | Baseline (I) – four weeks(VI) | 1239800.00000 | 140531.17092 | 62847.45023 | 1065307.50444 | 1414292.49556 | 19.727 | 4 | <0.001 | HS |
| Group B | Baseline (I) – four weeks(VI) | 1519300.00000 | 481648.24821 | 215399.64485 | 921254.71040 | 2117345.28960 | 7.053 | 4 | 0.002 | HS |
| Group C | Baseline (I) – four weeks(VI) | 1519900.00000 | 169860.25138 | 75963.81375 | 1308990.64113 | 1730809.35887 | 20.008 | 4 | <0.001 | HS |
| Group D | Baseline (I) – four weeks(VI) | 1439800.00000 | 96163.97454 | 43005.83681 | 1320396.65489 | 1559203.34511 | 33.479 | 4 | <0.001 | HS |
| Group E | Baseline (I) – four weeks(VI) | 1259700.00000 | 15779.89227 | 7056.98236 | 1240106.67587 | 1279293.32413 | 178.504 | 4 | <0.001 | HS |
HS – highly significant
*Paired t - test - Comparison of mean values of baseline values with the values at the end of 4 weeks of all the groups to evaluate their efficacy.
Results and Statistics
The results of the study were based on the number of colony forming units per milliliter [Table/Fig-2,4,5]. To evaluate the efficacy, the results of each group were subjected to paired t-test and to compare the efficacy of each disinfectant, data was subjected to one-way ANOVA.
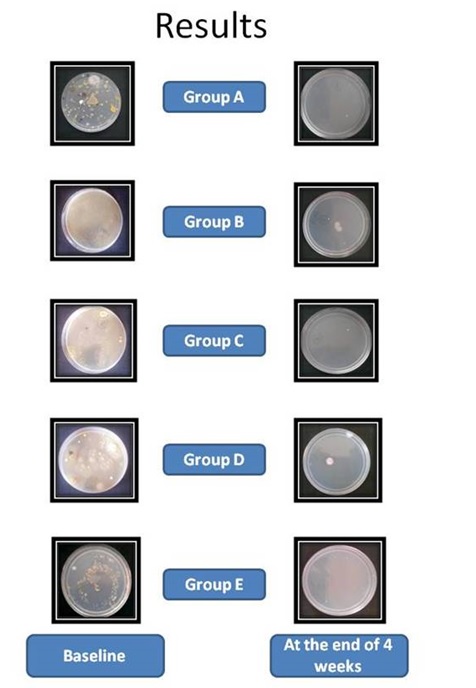
R2A agar culture plates of all the groups at baseline and at the end of 4 weeks.
Comparison of mean values of all the groups at different time intervals.
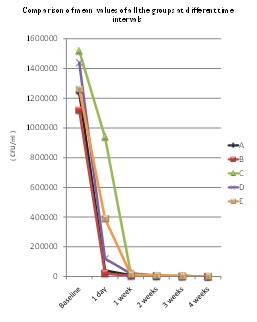
Paired t-test for each group showed that there was highly significant difference in mean values of subgroups II, III, IV, V & VI when compared with baseline values (subgroup I). It signifies that all the disinfectants were effective in controlling the microbial contamination of DUWL samples [Table/Fig-3]. Goals set by ADA & CDC were ultimately achieved at the end of 4 weeks.
When the data was subjected to one-way ANOVA no significant difference was found between the values of all the 5 groups tested therefore multiple comparison tests were not required [Table/Fig-2].
Discussion
Effective infection control is one of the cornerstones of good practice and clinical governance. The goal of infection control in dentistry is to reduce or eliminate exposure of patients and dental team members to microorganisms. Potential pathogens usually can come from patients and practitioners. Another source, however, could be from the environment, such as air or water [11]. Dentistry is unique since it is the only health care discipline that routinely uses tap water in the treatment of patients. In most cases, the water used comes from municipal utilities or from private tanks. Water moves directly into the dental units and then onto and through high-speed hand-pieces, 3-way (air-water) syringes, and power scalers enters into the oral cavity of patient [11]. Nobody would expect to have water expressed into their mouths that is less than drinking quality standard but despite this fact [12] it has been shown in numerous studies that untreated dental waterlines contain water that is highly contaminated.
For years, numerous attempts have been made, using various methods, focusing especially on the microbial contamination of water, to guarantee an appropriate quality of water used in dental treatment. Some well documented methods include nightly air purging of dental unit waterlines, flushing DUWL, independent bottled water systems which can be used in conjugation with filtration, anti-retraction devices, sterile water delivery system, chemical treatment with chemical agents [2]. This study evaluated various commercially available disinfectants in improving the microbiological quality of dental treatment water.
Hydrogen peroxide containing products are among most consistently effective disinfectants for DUWLs [13]. Hydrogen peroxide has been shown to possess a wide spectrum of antimicrobial activity. It is active against bacteria, yeasts, fungi, viruses and spores. The efficacy of hydrogen peroxide is multifactorial. It depends on concentration, pH, temperature, reaction time, use in combination with physical agents, further more it depends on other factors like bacterial/viral concentration, the microbial species under consideration and their biological phase (e.g., spore or vegetative status), the presence of organic substances. The nature of the surface to be treated (presence of pores, micro-cracks) and bacterial genetic properties also play a major role in efficacy of any disinfectant. The action of H2O2 on microbes is due to the presence of the hydroxyl radical (OH+) in the solution. The hydroxyl radical is said to be the strongest oxidant known. It can attack membrane lipids, DNA, and other essential cell components. Some of the biofilm-forming cells are killed by internally produced H2O2 [1].
In the present study 0.02% H2O2 was used as continuous disinfectant for 4 weeks. The mean baseline count was quiet high (1240000 CFU/ml). After disinfection regime there was drastic reduction in the microbial colony count in one day (40000 CFU/ml) and subsequent reduction was noticed in subsequent weeks. The resultant value after 4th week was 200 CFU/ml [Table/Fig-2]. It was found that 0.02% H2O2 when used continuously is effective in reducing the microbial count of DUWLs but the set goals by ADA & CDC were achieved only after 4 weeks of disinfection. The results were in accordance with the studies done by Walker JT et al., and Schel AJ et al., [14,15]. For a more rapid effect, 0.02% H2O2 was used continuously with additional shock treatment (0.25% H2O2) at the end of every week. The baseline counts (1120000 CFU/ml) were reduced rapidly after 1 day of disinfection and kept reducing progressively for following 4 weeks. However the results after 4 weeks (700 CFU/ml) were not in accordance with ADA & CDC guidelines. The microbial count value obtained in this regime may be due to resistance of certain strains of microbes to the disinfectant. The results of the current study were in agreement with a study carried out by Linger JB et al., who investigated the use of a hydrogen peroxide-based dental unit waterline treatment to reduce the colonization and growth of heterotrophic bacteria using the same methodology [16]. Similar results were reported by Orro G et al., Decoret D et al., and Szymanska J in their studies [1,17,18].
Antimicrobials used in oral rinses are commonly used to treat the familiar biofilm-dental plaque. Chlorohexidine gluconate is the best known bis-biguanide with a strong cationic charge and has a broad spectrum antibacterial activity. For centuries, the alcohols have been appreciated for their antimicrobial properties. For infection control purposes, ethyl alcohol (ethanol) and isopropyl alcohol (isopropanol) are the alcoholic solutions most often used. Their antimicrobial efficacies are enhanced in the presence of water, with optimal concentrations being from 60 to 90%, by volume. Antimicrobial action is due to coagulation (denaturation) of proteins, inactivation of enzymatic proteins, leading to the loss of specific cellular functions [19]. Overnight treatment with 0.12% Chlorohexidine and 12% ethanol was used for disinfection in the present study. Potent antibacterial effect of Chlorohexidine gluconate when used in conjugation with ethanol was demonstrated as the baseline count (1520000 CFU/ml) was reduced to 940000 CFU/ml after 1 day of disinfection regime. Further it reduced to 100 CFU/ml at the end of 4 weeks which were according to ADA & CDC guidelines. The results were in accordance with studies carried out by Porteous NB et al., Walker JT et al., Schel et al., Kettering J et al., Puttaiah R et al., Epstein JB et al., and Ozcan M et al., [6,14,15,20–23].
Another antimicrobial oral rinse, Original Listerine was also evaluated as DUWL disinfectant in the current study. It was used in 1:50 ratio with distilled water for overnight treatment of DUWLs. The baseline counts were 1440000 CFU/ml which reduced to 200 CFU/ml after the subsequent use of disinfectant for 4 weeks. The resultant values at the end of the study were in accordance with the guidelines laid down by ADA and CDC. Similar results were reported by Kettering J et al., and Meiller TF et al., in their respective studies [20,24]. Listerine consists of a mixture of three phenolic-derived essential oils: 0.064% thymol, 0.092% eucalyptol and 0.042% menthol combined with 0.060 methyl salicylate. Bacterial cell wall destruction, bacterial enzymatic inhibition, and extraction of bacterial lipopolysaccharides are the effects of Listerine on microorganisms which lead to their destruction [25].
Combination of 2% EDTA & 2% Sodium Perborate was used for 5 minutes daily in the morning. Baseline counts were 1260000 CFU/ml which reduced extensively after one day of disinfection regime and kept reducing for consecutive weeks. Microbial count after 4 weeks of disinfection was 300 CFU/ml. This formulation was effective in reducing the microbial count of DUWLs and results at the end of 4 weeks met the ADA & CDC guidelines. The results were in agreement with Montebugloni LL and Dolci GG [26]. This formulation can also be used as intermittent disinfectant in between patients. Besides keeping low level of heterotrophic bacterial counts during dental procedures, it could also be effective in eliminating oral pathogens eventually aspirated from patients under dental treatment.
Peracetic acid is one of the most powerful biocidal agent with a rapid and broad spectrum biocidal activity and could be a useful chemical for the purpose of controlling DUWL contamination although, as delivered, it has a series of side-effects. Preformed peracetic acid cannot be used directly as it is unstable, potentially explosive, highly acidic and as a consequence highly corrosive and these properties make products containing preformed peracetic acid difficult to formulate for long term storage stability and difficult to handle and transport so limiting the use of this product in dentistry. In recent years, a new chemical formulation (TetraAcetylEthyleneDiamine in association with persalt) has been proposed as a non hazardous means of generating peracetic acid in situ in the absence of preformed peracetic acid side-effects [26].
The results of the study showed that all the disinfectants as well as disinfection procedures successfully reduced the microbial colony count of DUWLs samples. ADA & CDC goals were achieved at the end of 4 weeks except in one group, which was very near to the goal and would have further reduced colony count in next couple of days. No significant difference was observed in efficacy of all the disinfectants and disinfection procedures used [Table/Fig-2]. Therefore, these disinfectants can be used effectively in daily clinical practice to improve the quality of dental unit water and to control infection.
According to current knowledge, it is not the mere presence of bacteria in Dental Unit Water Lines, also their number & type is equally important. Limitations of this study were that micro-organisms were not identified and the biofilm producing and adherence properties of micro-organisms were not under the scope of this study. Therefore further studies can be carried out in areas like identification of micro-organisms, seasonal variations in quality of DUWL water samples and effect of disinfectants on biofilm present in DUWLs by means of invivo or invitro. This will help in fabrication of more effective disinfection procedures to improve the quality of dental treatment water.
Conclusion
Within the limitations of the current study it can be concluded that all the DUWLs are heavily contaminated with microbes and pose potential risk both to the patient as well as the DHCPs. Both intermittent and continuous methods of disinfection considerably reduce the microbial counts of DUWLs. After 4 weeks of disinfection regime all the disinfectants effectively reduced the microbial colony count in accordance with ADA & CDC guidelines except group B, which was close enough to the guidelines. All the disinfectants and disinfection procedures were equally effective in reducing the microbial colony count of DUWLs at the end of 4 weeks.
The contamination of DUWLs is an issue that now concerns the dental profession on a number of levels, since patients and staff are regularly exposed to water and aerosols generated from the dental unit. The results of present study show the importance of routine monitoring of microbiological contamination of dental units and regular use of disinfection procedures to improve the microbial quality of dental unit water.
*Non Significant
HS – highly significant
*Paired t - test - Comparison of mean values of baseline values with the values at the end of 4 weeks of all the groups to evaluate their efficacy.