Chronic inflammatory periodontal diseases (gingivitis/periodontitis) are one of most common pathologies found in the oral cavity worldwide [1,2]. From the time of the Sumerians i.e., for nearly 5,000 years, calculus was considered to be the prime etiologic agent for these periodontal diseases [3]. From that period to almost 1920s, very little was known about the etiopathogenesis of periodontal diseases [4]. Ever since late 19th century, calculus has been deposed by plaque, and the hardened criminal i.e. calculus has come to be viewed as a fossilized remnant of minor significance [3]. As the concepts of etiopathogenesis of periodontal diseases have changed over time, dental plaque took the entire spotlight and calculus was almost ignored. Currently, dental plaque remains the mainstay in the etiology of periodontal diseases [5,6].
The current concepts of etiopathogenesis suggest that gingivitis is initiated due to the enzymatic effects and toxins released by the pathogenic microorganisms of the plaque. It is known that retention of plaque close to the gingiva is hastened by formation of supragingival dental calculus. This leads to plaque induced gingivitis which eventually progress to periodontal pockets. The formation of periodontal pockets in turn leads to deposition of sub-gingival calculus and promotes the disease chronicity. Therefore, deposition of calculus is considered to be only a secondary phenomenon and not an etiology [3]. Thus, according to these concepts calculus is considered as a mere predisposing and retentive factor since it does not irritate gingiva directly. It is always covered by a layer of microbial plaque which contains pathogenic microorganisms; which is a primary irritant and primary etiologic agent [5].
Although, some investigators accepted the fact that the formation of calculus is preceded by plaque formation therefore, they no longer had an issue about calculus not being a primary etiologic agent of periodontal diseases; however, they could not accept calculus merely as an ash heap. Adding volume to their thoughts, various morphologic and analytical studies pointed out porous nature of calculus, leading to retention of bacterial antigens and readily available toxic stimulators of bone resorption [3].
Furthermore, various Transmission Electron Microscopic studies have proved the presence of intact and viable bacteria within the non mineralised channels and islands in supra-gingival calculus and bacterial cultures have been reported in various microbiological studies on calculus [7,8]. However, the viability of bacteria in dental calculus and their role in the pathogenesis and disease progression still remains an enigma.
The present study brings dental calculus back into the spotlight and explores the field which is least touched, revealing the facts which are still hidden i.e., the viability of bacteria in dental calculus. To the author’s best knowledge, there is no classification of dental calculus in literature according to the extent of mineralization. Since, the role of microorganisms in mineralization of calculus is already established and there is change in microflora as the transition from plaque to calculus occurs [9–11]. It is also a well known fact that rate of calculus formation can vary among individuals [9–11]. Therefore, in the present study the calculus was categorized according to amount of mineralization, rather than the time of mineralization and described the terms highly mineralized, moderately mineralized and less mineralized calculus. This categorization of the calculus samples was done on the basis of force required to crush the samples.
The present study was undertaken with the aim to investigate the viability of bacteria in the dental calculus and to identify the same. The objectives were to observe the motility of bacteria under dark field microscope, to identify the bacterial morphotypes using gram staining, to obtain the bacterial cultures under aerobic and capnophillic conditions, to observe and identify the bacteria obtained in culture using varying biochemical assays.
Materials and Methods
For this prospective, observation based study, after obtaining institutional ethical committee clearance, a total of 30 patients with chronic inflammatory periodontal disease in 15-60 years of age group with equal distribution of chronic gingivitis and periodontitis were chosen. Exclusion criteria of patients was presence of any systemic disease, any salivary gland disease and/or xerostomia, antimicrobial therapy since past six months, patients who underwent oral prophylaxis for at least six months prior to harvesting the samples, pregnant and lactating women. After taking informed consent, two samples of supra-gingival calculus from lingual surfaces of mandibular anterior teeth of each patient were harvested knowing the fact that this is the site where the greatest amount of supra-gingival calculus is present [9,12].
The calculus samples were harvested as a single large piece. Thus, a total of 60 samples were obtained which were further divided into Group A and Group B.
Upon procurement, Group A (30 samples) were immediately placed in normal saline in sterile test tubes and Group B (30 samples) immediately kept in sterile dry glass vials. As calculus is always covered with a layer of plaque; thus plaque layer of Group A samples was kept intact and therefore acted as a control group. The samples in dry vials were irradiated in Laminar Air Flow Station {Toshiba (India) 3x 2x 2H, Hp1/4, volts220/230} using UV germicidal tube (253.5nm wavelength) for half an hour with intermittent turning of vials to ensure complete irradiation of calculus sample [7,8]. This was done to eliminate the surface contamination i.e., plaque, keeping the microorganisms inside the calculus intact. Thus, Group B samples i.e., irradiated calculus samples were the test samples in the study in which the viable microorganisms were to be determined and identified.
Both the Group A and Group B samples were separately crushed between two sterile microscopic glass slides (Bluestar Microslides, Polar Industrial Corporation, Mumbai, India) by applying thumb force. This thumb force required to crush each sample was recorded by using Electronic weighing scale (Mars Digital Scales and Systems, Delhi, India) in Newton units (N).
Based on the Newton units recorded, the irradiated calculus samples (Group B) were categorized into three subgroups- highly mineralized, moderately mineralized and less mineralized with 10 samples in each sub-group.
Samples were described as less mineralized, if less than 45 N force was required to crush the calculus samples. If 45-98 N force was required to crush the calculus samples, they were termed as moderately mineralized. Calculus samples which required more than 98 N force was required to be crushed were categorized as highly mineralized.
Irradiated and non-irradiated crushed samples (Group A and B) were divided into three parts in separate sterilized tubes. First portion was used for dark field microscopic examination for which one drop of calculus suspension was transferred on a microscopic glass slide, mounted with coverslip using normal saline. This was viewed under dark ground microscope (Olympus BX41 System Microscope) under oil immersion objective [100X] using liquid paraffin for the presence of spirochaetes and their motility.
A smear was prepared from the second portion and gram staining was done. This was observed under oil immersion objective [100X] using liquid paraffin oil.
Third portion of samples were vortexed for 2 minutes and 100μl was transferred using autopipette (Vertex Micropippete, RV Instruments Pvt. Ltd., 5l) separately into two tubes containing 1.5ml BHI broth (Brain Heart Infusion Broth, Himedia Laboratories Pvt. Ltd., Mumbai) and incubated for 48 hours at 37°C (Bacteriological Incubator, Universal Navyug, India).
One loopful of these BHI broth samples from non-irradiated and irradiated calculus samples were inoculated on the surface chocolate and Mac Conkey Agar and incubated under aerobic and capnophilic conditions at 37°C for 48 hours. The bacterial colonies thus obtained were identified on the basis of standard identification protocols and biochemical assays [7]. All the findings obtained from this observation-based study were recorded at each step and were tabulated systematically. These findings were then compared using Microsoft Excel 2007.
Results
Dark-field microscopic examination [Table/Fig-1a,b] of all samples of Group A revealed presence of spirochetes, which were moderate in number and slowly motile in majority of samples. Whereas, 28 samples of Group B showed presence of spirochetes which were scanty and sluggishly motile in majority of samples and two samples showed no organism.
Photomicrograph of dark field microscopic examination of dental calculus showing presence of motile spirochetes [100 X magnification].
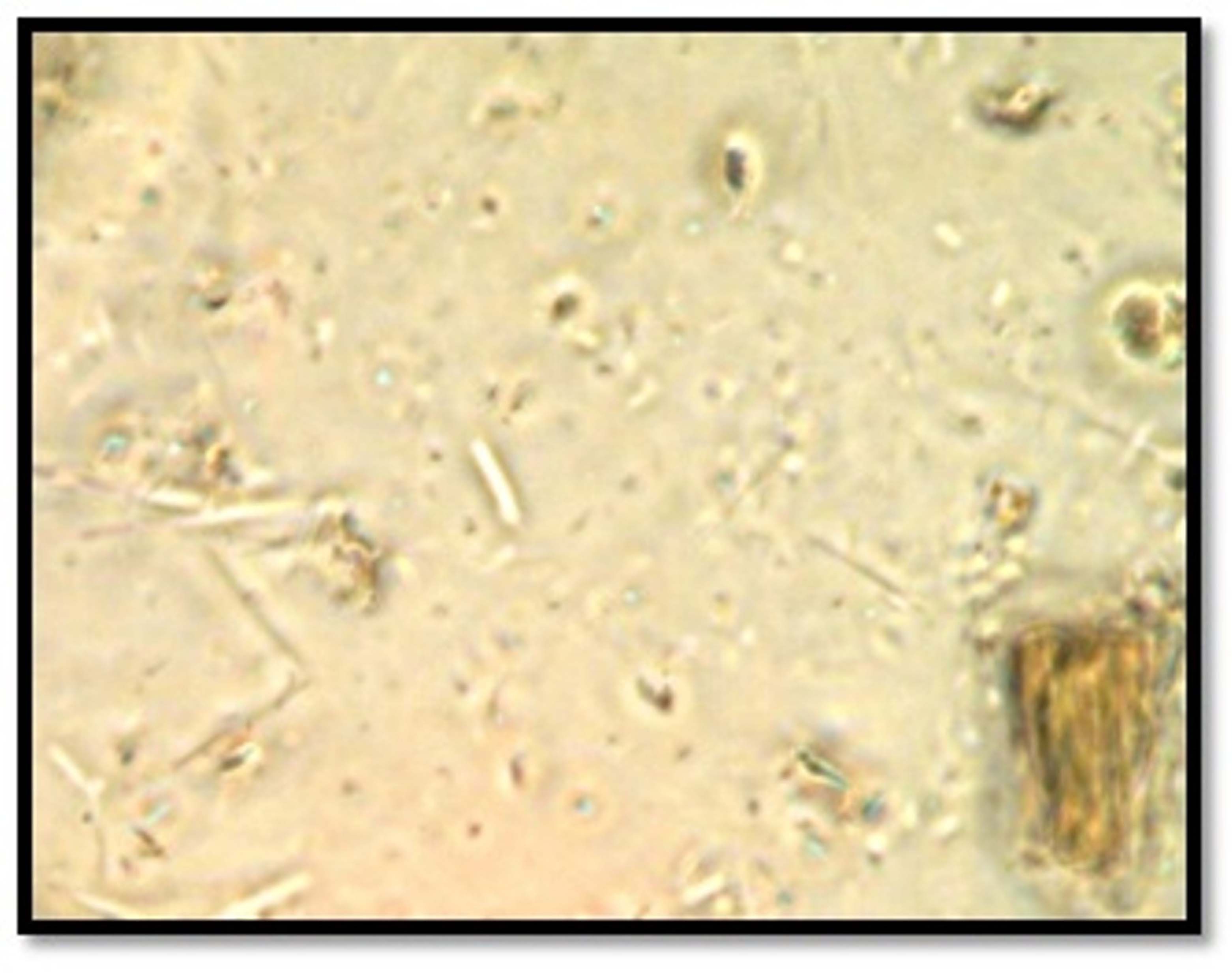
Findings of dark-field microscopic examination of calculus samples.
| Group A | Group B |
|---|
| | Highly Mineralized | Moderately Mineralized | Less Mineralized | Gingivitis | Periodontitis |
|---|
| Total samples | 30 | 30 | 10 | 10 | 10 | 15 | 15 |
| Large in number and actively motile spirochaetes | 8 | 1 | 0 | 1 | 0 | 0 | 1 |
| Moderate in number and slowly motile spirochaetes | 21 | 3 | 1 | 3 | 0 | 0 | 3 |
| Scanty in number and sluggishly motile spirochaetes | 1 | 24 | 9 | 4 | 10 | 14 | 10 |
| No organisms | 0 | 2 | 0 | 2 | 0 | 0 | 1 |
When dark-field microscopic findings of irradiated crushed calculus samples (Group B) categorized on basis of degree of mineralization were compared [Table/Fig-1a, b], all less mineralized irradiated crushed calculus samples showed scanty and sluggishly motile spirochaetes. In moderately mineralized irradiated crushed calculus samples, eight samples showed presence of spirochaetes which were scanty and sluggishly motile spirochaetes in maximum samples and two showed no organisms. All the highly mineralized irradiated crushed calculus samples showed presence of spirochaetes which were scanty and sluggishly motile in majority of samples.
On comparing dark-field microscopic findings of irradiated crushed calculus samples (Group B) harvested from gingivitis patients with that of samples harvested from periodontitis [Table/Fig-1b], it was found that out of 15 samples harvested from gingivitis patients 14 showed presence of spirochaetes which were scanty and sluggishly motile in majority of samples, and only one sample showed no organism. Similar results were observed in calculus samples harvested from periodontitis patients.
Almost similar gram staining characters were observed in Group A and B samples. Both showed gram positive cocci and gram negative bacilli. The samples of less, moderate and highly mineralized dental calculus in the present study showed almost similar observations. Almost similar gram staining characters were observed in the irradiated dental calculus samples harvested from patients of gingivitis and periodontitis [Table/Fig-2a,b].
Photomicrograph of gram stained crushed dental calculus samples showing gram positive cocci and gram negative bacilli [100 X magnification].
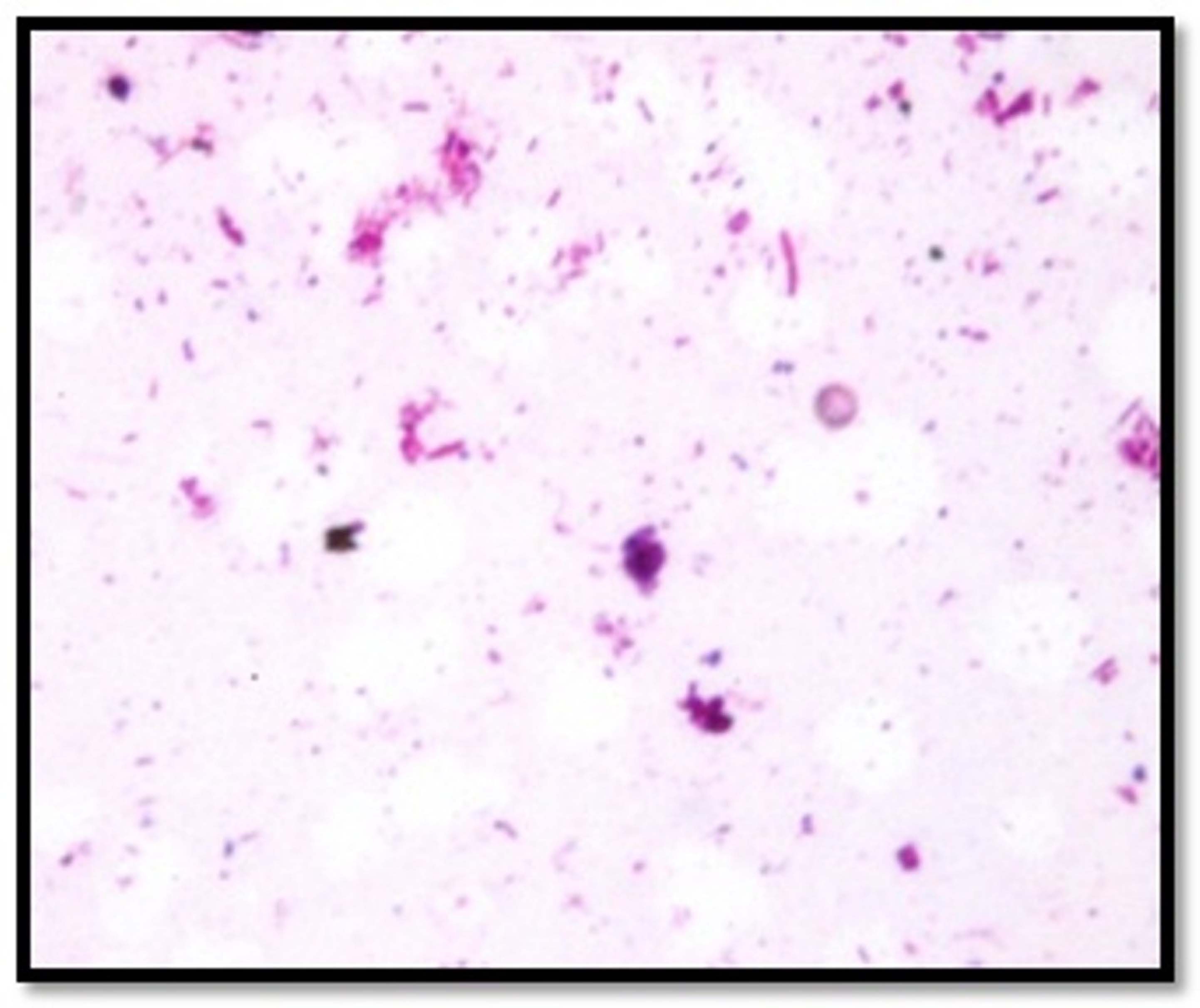
Gram staining characters observed in calculus samples.
| Group A | Group B |
|---|
| | Highly Mineralized | Moderately Mineralized | Less Mineralized | Gingivitis | Periodontitis |
|---|
| Total samples | 30 | 30 | 10 | 10 | 10 | 15 | 15 |
| Mixed organisms | 19 | 16 | 5 | 7 | 4 | 8 | 8 |
| Gram positive cocci | 4 | 4 | 3 | 1 | 0 | 0 | 4 |
| Gram negative bacilli | 7 | 7 | 0 | 2 | 5 | 6 | 1 |
| No organisms | 0 | 0 | 2 | 0 | 1 | 1 | 2 |
Bacterial cultures of calculus samples revealed growth in the entire Group A samples whereas one sample of Group B showed no growth and 29 samples revealed bacterial growth [Table/Fig-3a,b]. However, Group A and B exhibited almost similar growth. Growth of coagulase positive and coagulase negative Staphylococcus sp., Viridans streptococci, Klebsiella, Escherichia coli, Lactobacilli, Pseudomonas was observed in both irradiated and non-irradiated calculus samples. Additional growth of Proteus was observed in Group B samples. In spite of little variation in the microorganisms observed in less/moderately or highly mineralized calculus samples or the samples harvested from gingivitis/periodontitis; the predominant microorganisms found were coagulase negative and positive Staphylococcus sp. and viridians streptococci [Table/Fig-3a,b].
Bacterial cultures obtained from crushed dental calculus samples on chocolate agar [a-f] growth of Klebsiella sp.[a], Lactobacilli sp.[b], Staphylococcus sp. [c], Proteus sp. [d], Pseudomonas sp. [e], Viridans streptococci [f] and on Mac Conkey agar [g-l] showing growth E.coli [g], Klebsiella sp. [h,i], Lactobacilli sp. [j], Pseudomonas sp. [k], Staphyloccucus sp.[l].
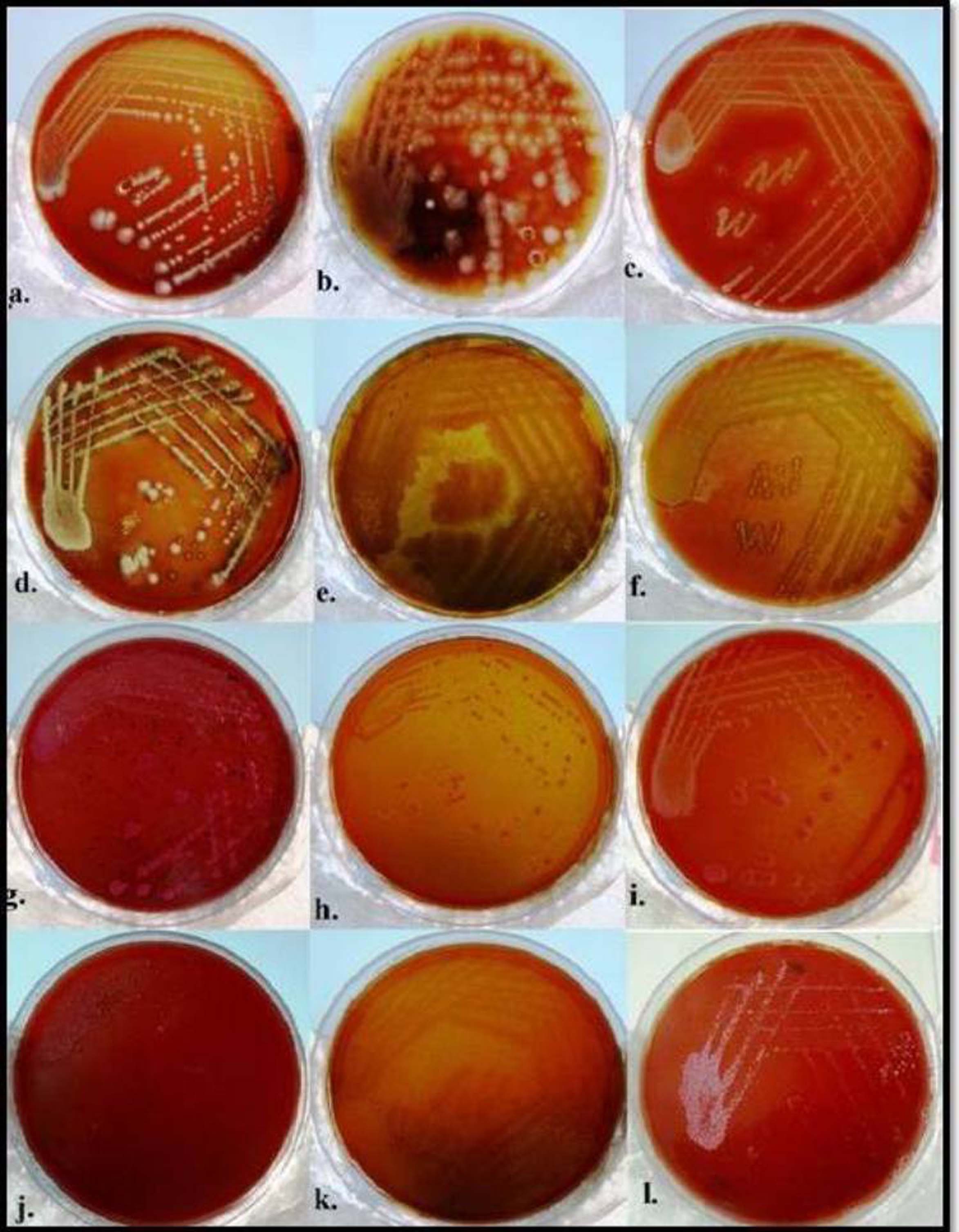
Bacterial culture growth obtained from calculus samples on chocolate and Mac Conkey agar under aerobic and capnophilic conditions and its subsequent identification.
| Group A | Group B |
|---|
| | Highly Mineralized | Moderately Mineralized | Less Mineralized | Gingivitis | Periodontitis |
|---|
| Total samples | 30 | 30 | 10 | 10 | 10 | 15 | 15 |
| Number of samples showing growth | 30 | 29 | 9 | 10 | 10 | 15 | 14 |
| No growth | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Coagulase negative Staphylococci | 4 | 9 | 4 | 1 | 4 | 5 | 4 |
| Coagulase positive Staphylococci | 12 | 9 | 2 | 5 | 2 | 5 | 4 |
| Viridans streptococci | 13 | 11 | 4 | 5 | 2 | 3 | 8 |
| Lactobacilli | 1 | 2 | 0 | 1 | 1 | 2 | 0 |
| Pseudomonas | 5 | 1 | 0 | 0 | 1 | 1 | 0 |
| E. coli | 3 | 3 | 0 | 3 | 0 | 1 | 2 |
| Klebsiella | 7 | 8 | 3 | 1 | 4 | 5 | 3 |
| Proteus | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
Discussion
The highlight of present study is that it attempted to research upon the effects of degree of mineralization on the viability and type of organisms present in dental calculus. Also, it has studied the difference between the viability and type of bacteria observed in dental calculus in patients with gingivitis and in patients with periodontitis. To the best of our knowledge, no research study is published till date which has taken these factors into consideration and researched upon.
Spirochetes are one of the most common microorganisms associated with periodontal diseases. Dark-field microscopy is an ideal tool to demonstrate the presence and motility of spirochaetes as they are difficult to be cultured [8]. In the present study, all the non-irradiated samples (Group A) revealed the presence of spirochaetes in all the samples and moderate numbers of slowly motile spirochaetes in majority of samples. In contrast, 28 samples of irradiated calculus (Group B) revealed presence of spirochaetes and majority showed scanty and sluggishly motile spirochaetes and only two samples showed no organisms.
To our best knowledge, there are only two studies in English literature by Moolya et al., and Kaur et al., in which the dark-field microscopic examination of dental calculus samples was done [8,13]. Similar to the present study, both these studies revealed the presence of spirochetes in all the calculus samples examined. However, the present study also commented on the number and degree of motility of spirochetes which has not been conducted by any published study till date.
Further, there was very little variation in numbers and degree of motility of spirochetes in the crushed irradiated calculus samples categorized according to degree of mineralization or according to degree of disease severity (gingivitis/periodontitis). However, majority of samples showed presence of scanty and sluggishly motile spirochetes.
The morphology of microorganisms can be studied by gram staining. The importance of the same lies in the fact that gram positive and gram negative bacteria differ not merely in staining characteristics and in structure but also in other properties such as growth requirements, susceptibility to antibiotics and pathogenecity [8,13].
The present study revealed almost similar gram staining characters in irradiated and non- irradiated calculus samples. All non- irradiated (Group A) samples and 27 irradiated (Group B) samples showed gram positive cocci and gram negative bacilli. Only three of all irradiated samples showed no organisms.
Literature search revealed two studies by Moolya et al., and Kaur et al., which have performed gram staining on dental calculus [8,13]. The results of the present study are in accordance with Moolya et al., [8]. The latter found presence of gram positive cocci and gram-negative bacilli. However, Kaur et al., found no presence of gram positive cocci in their study and observed gram-negative bacilli, gram-negative cocci and filamentous microorganisms [13]. As in the latter study, the samples harvested were both supra-gingival and sub-gingival without elimination of overlying plaque. Thus, there can be possibility that either surface calculus with plaque which generally contains a greater proportion of filamentous microorganisms or sub-gingival plaque which is dominated by gram negative bacilli was studied by them [14,15]. In the present study, subsequent successful bacterial culture of gram positive cocci i.e., coagulase negative and positive Staphylococcus sp., viridians streptococci were also obtained.
Further, the irradiated samples of less, moderate and highly mineralized dental calculus in the present study showed almost similar observations. Also, almost similar gram staining characters were observed in the irradiated dental calculus samples harvested from patients of gingivitis and periodontitis.
Obtaining successful bacterial culture is an important sign of bacterial viability. In the present study, bacterial cultures were performed under aerobic and capnophilic conditions. All 30 non-irradiated Group A samples showed growth in bacterial cultures. However, 29 of the irradiated Group B samples showed growth while one showed no growth. The probable reason for this no growth could be that surface microorganisms from calculus samples were killed as they were exposed to UV irradiation. Additionally, there may be a possibility that no viable bacteria were present inside it.
There are very few studies in literature on dental calculus which have performed bacterial cultures in order to investigate bacterial viability within dental calculus. Successful bacterial cultures from supra-gingival and sub-gingival calculus were reported by Sidaway however the superficial covering of plaque was not eliminated in their study [16]. Similar to the present study, Tan et al., also used the same method of exposing dental calculus to UV irradiation overnight to eliminate overlying plaque [7]. In their study successful cultures in five out of seven calculus samples were reported and two showed no growth. Similarly, Moolya et al., exposed calculus samples to UV radiation for half an hour and revealed bacterial culture growth in all irradiated samples [8].
The observations of present study revealed the growth of coagulase positive and coagulase negative Staphylococcus sp., viridans streptococci, Klebsiella, Escherichia coli, Lactobacilli, Pseudomonas in irradiated and non- irradiated calculus samples. The highlight of present study is that it reports successful cultures of microorganisms from dental calculus viz. Klebsiella, Escherichia coli, Lactobacilli and Proteus which, to our best knowledge have not been reported in any other study till date.
Furthermore, inspite of little variation in the microorganisms observed in less/moderately/highly mineralized calculus samples or the samples harvested from gingivitis/periodontitis; the predominant microorganisms always remained to be coagulase negative and positive Staphylococcus sp. and Viridans streptococci. This is in accordance with the study conducted by Moolya et al., (results of latter have been described earlier), and the ultrastructural study by Tan et al., in which gram positive cocci with appearance similar to Staphylococci sp. were observed inside non-mineralized channels or islands within dental calculus [7,8].
Thus, dark-field microscopic examination and bacterial cultures in the present study clearly revealed the presence of viable bacteria within dental calculus. Although, the literature implies that calculus may be essentially mineralized dead organic material, however; microradiographic and electron microscopic studies of formed calculus have shown that variety of gram-positive and gram negative micro organisms becomes calcified when they are immersed in calcium phosphate solution [13].
Therefore, there may be a possibility that some of the viable microorganisms readily calcify whereas others may not. This eventually leads to formation of pockets containing non-mineralized viable bacteria within calcified substance. Thus, calculus may act as a reservoir of viable pathogenic microorganisms in the oral cavity. This reservoir may play a crucial role in etiology of periodontal diseases [7,8,17]. As described by Mandel and Gaffar calculus is a "toxic dump waste" and also called as a "slow releasing device releasing toxic and pathogenic products into the soft tissues" [3,7] . Thus, it may be possible that if viable bacteria are present within the substance of calculus then, they may release toxic antigenic metabolites that may leach out from the calculus. If the bacteria are non-vital, then their degradation would release toxic byproducts e.g. lipopolysaccaride remnants, which would be leached out of the calculus. These leached out products may initiate the inflammatory responses into soft tissues [3,7].
The present study provides strong evidence that viable microorganisms reside within dental calculus presumably, within lacunae and channels. Furthermore, the results of the culture data imply that some of these bacteria are capable of growth when placed in suitable environment, as might be the scenario with incomplete removal of calculus/fracture of calculus deposits while brushing [7]. This may lead to exposure of these microorganisms which may lead to development of diseases.
Future directions: It is probable that high prevalence of calculus and the focus of dental professionals on calculus removal will demand further research by academicians considering the fact that present study provides a strong evidence of viability of bacteria in calculus and thus its pathogenic nature. The observations of the present study could be further elucidated by including sub-gingival calculus or by performing anaerobic cultures on larger sample size giving a clear picture of microbiota present within dental calculus.
This study may be a roadway for various studies such as virulence and behaviour of bacteria in dental calculus, their pathogenecity, effect of age, sex, habits on microbiota of dental calculus etc., to help in solving the puzzle of the pathogenesis of periodontal diseases.
Limitation
The shortcoming of the study is its limited sample size. Also, this study gives an idea about the aerobic and capnophilic microorganisms only. However, anaerobic microbiota is also important for disease pathogenesis. Therefore, further research should be aimed at performing anaerobic cultures analyzing a larger sample, including not only age and gender but other parameters also such as racial and cultural differences.
Conclusion
The findings of present study indicate that calculus can act as a reservoir of viable pathogenic microorganisms; thus may play an important role in etiopathogenesis and progression of periodontal diseases. This is in contrast to the earlier reports suggesting that calculus being completely mineralized therefore was considered to be a mere predisposing factor in pathogenesis of periodontal diseases.