Quantitative Gene Expression of ERG9 in Model Saccharomyces cerevisiae: Chamomile Extract For Human Cancer Treatment
Maryam Hosseinpour1, Mohsen Mobini-Dehkordi2, Hossein Teimori3
1 Faculty of Science, Department of Genetics, University of Shahrekord, Shahrekord, Iran.
2 Faculty of Science, Department of Genetics, University of Shahrekord, Shahrekord, Iran.
3 Assistant Professor, Department of Genetics and Biotechnology, Cellular and Molecular Research Center, Shahrekord University of Medical Sciences, Shahrekord, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Hossein Teimori, PhD in Medical Genetics, Associate Professor Department of Medical Genetics, Shahrekord University of Medical Sciences, Rahmatiyeh, Shahrekord, Iran.
E-mail: hteimori@skums.ac.ir
Introduction
Over expression of squalene synthase gene causes induction of growth tumour and reduction of apoptosis. This gene which is conserved between Saccharomyces cerevisiae yeast and humans, is named (ERG9).
Aim
In this work, we studied the effect of Matricaria recutita extract on ERG9 gene (squalene synthase) expression in S.cerevisiae which was used as organism model in cancer therapy.
Materials and Methods
S. cerevisiae was cultured in YPD medium plus 0,250, 1000 and 3000 μg/ml of Matricaria recutita extract and we evaluated the (ERG9) gene expression by Real-time RT-PCR method after 24 hours.
Statistical analysis used
At least 3 independent experiments were done. Data were analyzed using One-way ANOVA and Dunnett’s test. A p-value of less than 0.01 was considered as significant.
Results
We found that 250, 1000 and 3000 μg/ml of Matricaria recutita extract could reduce expression of ERG9 gene significantly (p<0.01). Interestingly, the expression of this gene was completely inhibited in 1000 and 3000 μg/ml concentrations.
Conclusion
This study predicted that Matricaria recutita extract produced anti-cancer effects in humans, because it could inhibit the expression of an analogue key gene in this malignant disease. Further investigations should be made, to study its molecular mechanism of action at the mammal cell level.
Hydro alcoholic extract, Matricaria recutita, Medicinal plants
Introduction
Matricaria chamomilla (syn: M. recutita), commonly known as Chamomile or German Chamomile [1] is a valuable plant which belongs to the Asteraceae family, which has been used in herbal medicine for treatment of wounds, eczema, gout, neurological disorders, smallpox and other ailments. Extract of this plant has antioxidant, antimicrobial, and mild astringent properties [2]. Research done on animals suggests that it has anti-inflammatory [3], anti-allergic, anti-hyperglycaemic, antiulcer, antipruritic [4], anti-mutagenic and cholesterol-lowering effects [5]. Chamomile has been used to cure different gastrointestinal disturbances such as stomach upset, flatulence, motion sickness, anorexia, nausea, vomiting, diarrhoea and constipation or a combination of both [6]. Infections of parasitic worms, malaria, colds and flu symptoms can be treated with essential oil of Chamomile. In addition, Chamomile extract inhibits the growth of normal cells slightly, but this results in a significant reduction in the viability of a variety of cancer cell lines [7]. In addition, hydro alcoholic extract of this plant produces an anti-proliferative effect on yeast cells [8]. Biological activities of this plant are related to various classes of bioactive compounds which are present in it. Chamomile flowers contain more than 120 bioactive compounds. The terpenoids, α-bisabolol and its oxides and azulenes are the major constituents of this plant. Various therapeutically and biologically active agents have been identified to be present in it, including sesquiterpenes, flavonoids, coumarins and polyacetylenes [4]. There are many Phenolic compounds in extracts of Chamomile flowers, like the flavonoids apigenin, patuletin, quercetin, luteolin [9]. Although previous studies have demonstrated that Chamomile extract produces anti-proliferative effects on yeast and various human cancer cells, no study has yet been conducted to investigate its molecular mechanism in an appropriate eukaryotic model. In this regards, medicinal plants have mostly been used for a long period of time [10,11] and they have shown to be a relatively safe and reliable source for preparation of new drugs [12,13]. Investigations done on medicinal plants, especially on their mechanisms of action, help in improving their actions.
Here, the molecular mechanism of action of hydro alcoholic extract of Chamomile was studied, to evaluate the potential usefulness of this medicinal plant in treatment of human cancers.
Materials and Methods
This experimental study was conducted in Cellular and Molecular Research Center of Shahrekord University of Medical Sciences (Iran), over a period of 12 months from September 2013 to September 2014.
Plant materials and hydro alcoholic extract preparation: Approved M. chamomilla flowers were purchased from Goldarou Co. (Isfahan, Iran) and dried under shade at 37°C. The extraction was done by percolation method at 15-20°C, using ethanol 50%. One hundred grams of powdered chamomile was soaked in 1.2 l ethanol and the solution was transferred to a percolator for three days, filtered and evaporated at 37°C [14]. The dried material was kept at -20°C until it was used. Hydro alcoholic extract was dissolved in DMSO (Dimethyl Sulfoxide) (Sigma) to prepare stock solutions [7]. It was later added to culture media to achieve desired concentration.
Yeast and culture media: The yeast used in this study was Saccharomyces cerevisiae (PTCC 5052) cells which were purchased from Persian Type Culture Collection in Tehran, Iran. Yeast sample was kept at 4°C prior to culture. S. cerevisiae was cultured in the sterile, specific medium of yeast extract, peptone, dextrose (YPD) containing 2% glucose, 2% peptone and 1% yeast extract, and was incubated at 30°C for 48 h. After developing pure yeast suspension in broth medium, 5×107 cells were transferred to liquid medium containing no plant extract [(DMSO 1% as control), 250 μg/ml, 1000 μg/ml, and 3000μg/ml chamomile hydro alcoholic extract] and it was placed in an incubator shaker at 35°C for 24 h [8]. To evaluate the growth rate, we determined the optical density at 600nm using a spectrophotometer (DU 800 Beckman Coulter, CA, USA).
RNA extraction and cDNA synthesis: Total RNA was extracted according to the kit manufacture’s manual of Biozol Reagent (Bioplus). The RNA concentration and purity of each sample were measured by using Thermo Scientific NanoDrop2000 spectrophotometer. cDNA was synthesized by a two-step cDNA synthesis kit (Vivantis) based on the manufacturer’s protocol using random hexamers.
Primer design and Real-time polymerase chain reaction: The primer sets were designed by online Primer 3 software (http://www.broad.mit.edu/cgibin/primer/primer3). The following primers were used: TUB1: forward 5’CCAAGGGCTATTTACGTGGA3’, reverse 5’GGTGTAATGGCCTCTTGCAT3’ [15]; ERG9: forward 5’TGAAAGCATGGGTCTTTTCC3’, reverse 5’CAACCCCAGTTGTTCGTTTT3’. Each primer sequence was checked in the S. cerevisiae transcripttome using the Basic Local Alignment Search Tool (BLAST) to ensure detect single gene. The specifiicities of primers were verified by gel electrophoresis and melting curve analysis and the size of the amplicon was confirmed. Quantification of ERG9 gene expression was determined by real-time RT-PCR. Quantitative RT-PCR was performed using Rotor-gene 6000 (Corbett), thermo Scientific Maxima SYBR Green/ ROX Q PCR Mastermix (2X) kit and specific primers of ERG9 and TUB1, as an internal control, genes.
The PCR cycling conditions were as follows: 1 cycle at 95°C for 10 min (as an initial denaturation), 40 cycles of 95°C for 20s, 60°C for 20s and 72°C for 20s and a final extension step 72°C 5 min. Quantitative analysis of the genes in different groups was done by comparative Ct method (2−ΔΔCt, ΔCt = CtTUB1-CtERG9, ΔΔCt = ΔCtSample-ΔCtControl) [16].
Statistical Analysis
At least 3 independent experiments were done. Data were analyzed using One-way ANOVA, followed by Dunnett’s test using the SPSS software, version 18.0. The results were presented as mean ± Standard deviations (SD). A p-value less than 0.01 was considered as significant.
Results
Optimization of RT-PCR Reaction
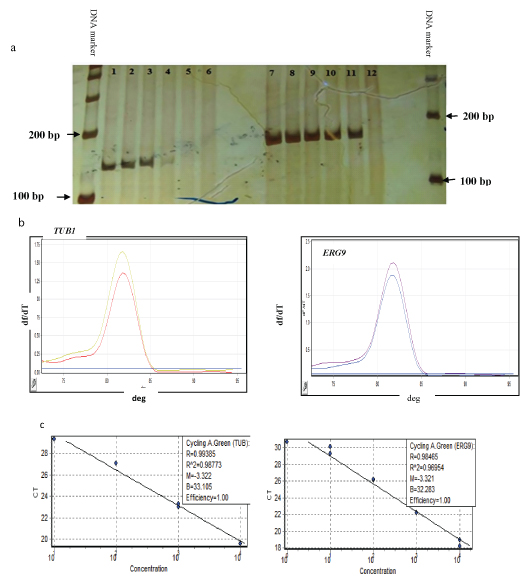
Optimizations for every set of primers were done by both conventional and quantitative RT-PCR reactions. Conventional PCR was performed with a temperature gradient and run on 8% polyacrylamide gel [Table/Fig-1a]. Melting curve analysis showed unique melting peaks without any primer-dimer formation [Table/Fig-1b]. PCR efficiency was confiirmed from the standard curve, from serial dilutions of the pooled cDNAs [Table/Fig-1c].
Optimization of real time -PCR reaction. (a) Single band of PCR product on polyacrylamide gel, 141bp fragment was amplified from TUB1 lines 1-5 and 157bp fragment was amplified from ERG9 lines 7-11, Lines 6 and 12: non-template control (NTC). (b) A specific melting curve without primer dimers for amplification on Real-Time PCR. (c) Standard curves of a cDNA dilution series for TUB1 and ERG9 showing the amplification efficiency near to 1.00.
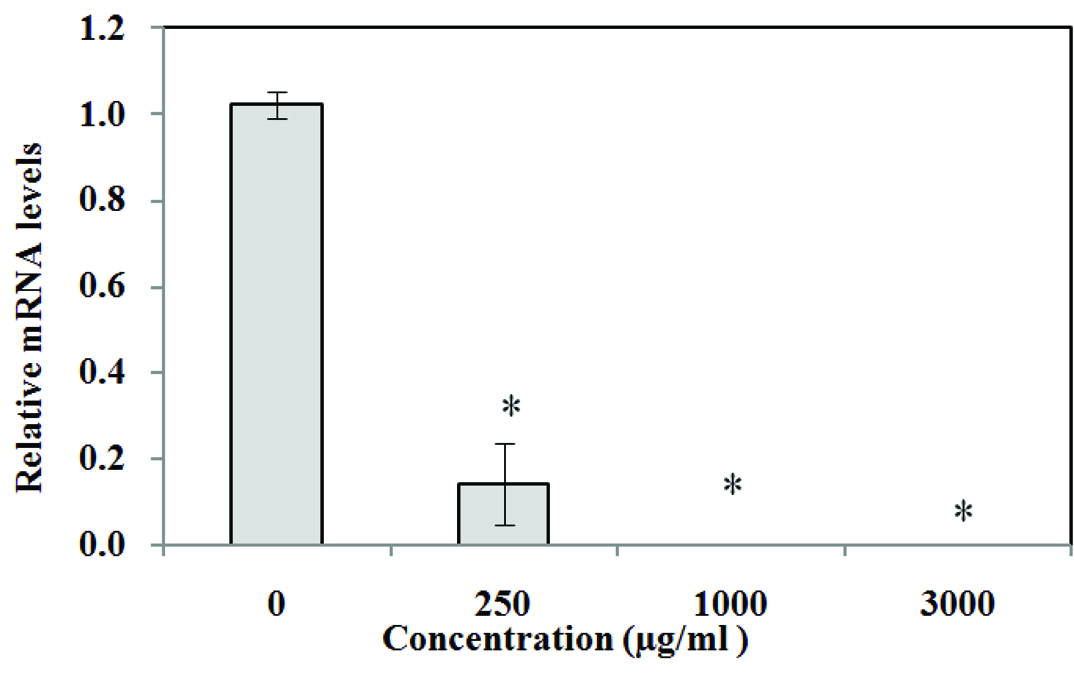
Expression of ERG9 gene in Yeast treated with Extract
The relative mRNA levels were determined by Real-Time PCR. TUB1 as the reference gene, was used to normalize the data of all samples.
The expression of gene at 250 μg/ml concentration extract was found to be decreased significantly as compared to the control group (p<0.01). At concentrations of 1000 and 3000 μg/ml, the expression decreased remarkably and no mRNA was detected by Rotor-Gene 6000 (Corbett) [Table/Fig-2].
Effect of Chamomile hydro alcoholic extract on expression of ERG9 gene in yeast; The medium containing 1% (V/V) DMSO was determined as negative control. Values are means ± SD (n = 3). p<0.01 versus control.
Discussion
Natural products are important sources of therapeutic agents which are safe and economical medicaments [17]. To understand the roles of these natural compounds, we need efficient and economical biological systems. S.cerevisiae is a suitable eukaryotic model system for drug discovery and cancer treatment. High conservation of many of the basic cellular and molecular processes and gene functions was the reasons for selecting S. cerevisiae as an organism model for different molecular analysis [18,19].
About 30% of known genes involved in human disease may have orthologous in the yeast [20–22]; one of these conservative genes being ERG9 encoding squalene synthase. The structure and reaction mechanism of Squalene Synthase (SQS) are markedly conserved throughout evolution [23,24].
SQS plays a crucial role in cholesterol biosynthesis and it catalyzes the first step of the sterol branch in the mevalonate/ isoprenoid pathway [24]. SQS activity and de novo cholesterol synthesis determine levels of cholesterol content of lipid rafts, membrane domain enriched in cholesterol and sphingolipids. Elevated levels of cholesterol content of lipid rafts are associated with enhanced tumour growth and reduced apoptosis [25]. Recent findings have showed that over expression enhances metastasis in lung cancer [26]. In our previous work, we showed that exposure of chamomile extracts caused a significant decrease in cell viability in S. cerevisiae [8] at 3000μg/ml concentration, but not at concentrations of 500, 1000 and 2000μg/ml; Here, we used this yeast as a model of a genetic organism, to evaluate the mechanism of action of chamomile; we found that chamomile extract significantly decreased ERG9 mRNA level at 250 μg/ml concentration, as compared to control. Interestingly, at concentrations of 1000 and 3000 μg/ml no mRNA was detected by Rotor-Gene 6000 (p<0.01) [Table/Fig-2]. This result showed that decrease or complete inhibition of ERG9 gene (250μg/ml and 1000μg/ml) could not decrease growth of yeast cells.
In Slusarz A et al., study done on anticancer effect of chamomile extract, the most important component of this extract, epigenin, which comprises 80% of extract is and its effect on the hedgehog signaling pathway was examined. The hedgehog signaling pathway is one of the most important pathways which increased signaling, contributed greatly to prostate cancer progression. Slusarz A et al., study indicated that epigenin decreased Gli1 (a transcription factor which causes activation of target genes in this pathway) mRNA concentrations [27].
Because Chamomile extract contains more than 120 bioactive compounds, it can cause not only reduction in the hedgehog signaling pathway by decreasing Gli1 gene expression, but it can also cause induced apoptosis and decrease growth of cancer cells by decreasing ERG9 gene expression.
Remarkably, as SQS knock down attenuates the invasion potential of lung and prostate cancer cells, similar effects can occur when cancer cells are treated with the SQS chemical inhibitor i.e. Zaragoza acid A. Therefore, SQS inhibitors may have considerable potential for antineoplastic intervention [25,26].
This study shows the decrease in ERG9 gene expression with Chamomile Extract treatment, but it does not show correlation with protein level or SQS activity of Squalene Synthases (SQS).
Hence, further attention should be given to the mechanism of action of chamomile extract at the mammal cell level and it is recommended that the effective substance of this extract on ERG9 gene expression should be identified.
Conclusion
This research demonstrates the molecular mechanism of the anti-proliferation action of chamomile extract. We found that chamomile extract significantly decrease ERG9 mRNA concentration; our findings on complete inhibition of ERG9 gene expression in the presence of Chamomile extracts concludes that it can be used as a prospective available, safer and more affordable anticancer agent.
[1]. Tolouee M, Alinezhad S, Saberi R, Eslamifar A, Zad SJ, Jaimand K, Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van TieghemInternational Journal of Food Microbiology 2010 139(3):127-33. [Google Scholar]
[2]. Pereira RP, Fachinetto R, de Souza Prestes A, Puntel RL, da Silva GNS, Heinzmann BM, Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratusNeurochem Res 2009 34(5):973-83. [Google Scholar]
[3]. Ganzera M, Schneider P, Stuppner H, Inhibitory effects of the essential oil of chamomile Matricaria recutita L. and its major constituents on human cytochrome P450 enzymesLife Sci 2006 78(8):856-61. [Google Scholar]
[4]. Gupta V, Mittal P, Bansal P, Khokra SL, Kaushik D, Pharmacological potential of Matricaria recutita-A reviewInt J Pharm Sci Drug Res 2010 2(1):12-16. [Google Scholar]
[5]. Petronilho S, Maraschin M, Coimbra MA, Rocha SM, In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of chamomile Matricaria recutita LInd Crops Prod 2012 40:1-12. [Google Scholar]
[6]. Srivastava JK, Shankar E, Gupta S, Chamomile: A herbal medicine of the past with bright futureMol Med Rep 2010 3(6):895 [Google Scholar]
[7]. Srivastava JK, Gupta S, Antiproliferative and apoptotic effects of chamomile extract in various human cancer cellsJ Agric Food Chem 2007 55(23):9470-78. [Google Scholar]
[8]. Hosseinpour M, Mobini-Dehkordi M, Saffar B, Hossein T, Antiproliferative effects of Matricaria chamomilla on Saccharomyces cerevisiaeJ Herb Med Pharmacol 2013 2(2) [Google Scholar]
[9]. McKay DL, Blumberg JB, A review of the bioactivity and potential health benefits of peppermint tea Mentha piperita LPhytotherapy Research 2006 20(8):619-33. [Google Scholar]
[10]. Rafieian-Kopaei M, Sewell RD, The history and ups and downs of herbal medicines usageJournal of Herb Med Pharmacology 2014 3(1) [Google Scholar]
[11]. Bahmani M, Zargaran A, Rafieian-Kopaei M, Saki K, Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest IranAsian Pacific Journal of Tropical Medicine 2014 7:S348-S54. [Google Scholar]
[12]. Shirzad H, Nasri H, Toxicity and safety of medicinal plantsJournal of Herb Med Pharmacology 2014 2(2) [Google Scholar]
[13]. Saki K, Bahmani M, Rafieian-Kopaei M, The effect of most important medicinal plants on two importnt psychiatric disorders (anxiety and depression)-a reviewAsian Pacific Journal of Tropical Medicine 2014 7:S34-S42. [Google Scholar]
[14]. Karbalay-Doust S, Noorafshan A, Dehghani F, Panjehshahin M, Monabati A, Effects of hydroalcoholic extract of Matricaria chamomilla on serum testosterone and estradiol levels, spermatozoon quality, and tail length in ratIran J Med Sci 2010 35(2):122-28. [Google Scholar]
[15]. Xiang L, Sun K, Lu J, Weng Y, Taoka A, Sakagami Y, Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genesBioscience, Biotechnology and Biochemistry 2011 75(5):854-58. [Google Scholar]
[16]. Wang J, Ni Z, Duan Z, Wang G, Li F, Altered expression of hypoxia-inducible factor-1α (HIF-1α) and its regulatory genes in gastric cancer tissuesPloS one 2014 9(6):e99835 [Google Scholar]
[17]. Lahlou M, The success of natural products in drug discoveryPharmacology & Pharmacy 2013 4:17-31. [Google Scholar]
[18]. Ma D, Applications of yeast in drug discovery. Progress in drug research 2001 Springer:117-62. [Google Scholar]
[19]. Auerbach D, Arnoldo A, Bogdan B, Fetchko M, Stagljar I, Drug discovery using yeast as a model system: a functional genomic and proteomic viewCurr Proteomics 2005 2(1):1-13. [Google Scholar]
[20]. Karathia H, Vilaprinyo E, Sorribas A, Alves R, Saccharomyces cerevisiae as a model organism: a comparative studyPloS one 2011 6(2):e16015 [Google Scholar]
[21]. Birrell GW, Giaever G, Chu AM, Davis RW, Brown JM, A genome-wide screen in Saccharomyces cerevisiae for genes affecting UV radiation sensitivityProceedings of the National Academy of Sciences 2001 98(22):12608-13. [Google Scholar]
[22]. Foury F, Human genetic diseases: a cross-talk between man and yeastGene 1997 195(1):1-10. [Google Scholar]
[23]. Robinson GW, Tsay Y, Kienzle BK, Smith-Monroy CA, Bishop RW, Conservation between human and fungal squalene synthetases: similarities in structure, function, and regulationMolecular and Cellular Biology 1993 13(5):2706-17. [Google Scholar]
[24]. Nakashima T, Inoue T, Oka A, Nishino T, Osumi T, Hata S, Cloning, expression, and characterization of cDNAs encoding Arabidopsis thaliana squalene synthaseProceedings of the National Academy of Sciences 1995 92(6):2328-32. [Google Scholar]
[25]. Brusselmans K, Timmermans L, Van de Sande T, Van Veldhoven PP, Guan G, Shechter I, Squalene synthase, a determinant of Raft-associated cholesterol and modulator of cancer cell proliferationJ Biol Chem 2007 282(26):18777-85. [Google Scholar]
[26]. Yang Y-F, Jan Y-H, Liu Y-P, Yang C-J, Su C-Y, Chang Y-C, Squalene synthase induces tumour necrosis factor receptor 1 enrichment in lipid rafts to promote lung cancer metastasisAmerican Journal of Respiratory and Critical Care Medicine 2014 190(6):675-87. [Google Scholar]
[27]. Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancerCancer Res 2010 70(8):3382-90. [Google Scholar]