Plaque is the primary etiological factor in gingival inflammation. Lack of proper maintenance of oral hygiene measures result in formation of pathogenic plaque [1]. Therefore, plaque control represents the cornerstone of good oral hygiene practice [2]. Majority of patients might not use these mouthrinses as a oral hygiene product effectively owing to the degree of awareness and motivation required [2]. Hence, to improve the potential deficiencies of daily self performed oral hygiene regime an adjunctive chemical plaque control approach is desirable.
Mouthrinses are the most frequently used chemical plaque control at home and are in use for centuries as breath fresheners, medicaments, antiseptics and can be used as a vehicle to deliver anti-plaque ingredient in the oral cavity for plaque control [3]. Normally, a therapeutic mouthrinse contains an active ingredient [4].
Chlorhexidine is regarded as the ‘gold standard’ anti-plaque agent and is particularly effective against oral biofilm [5] whereas Fluoride has been primarily utilized as an anti-caries agent. The use of fluoride mouthrinses is probably one of the most commonly used method for caries prevention [5].
Studies documenting the anti-plaque efficacy of mouthrinse containing chlorhexidine with fluoride are limited [6,7]. Studies for long term use of mouthrinses are rare which can produce a drop in plaque pH which may have a detrimental effect on oral hard tissues. Hence, the present study was undertaken with the aim to assess the anti-plaque efficacy of Chlorhexidine combined with Fluoride mouthwash and to measure its impact on plaque accumulation and plaque pH.
Materials and Methods
The present study was a double-blind, concurrent parallel, randomized clinical trial conducted for a period of seven days. A study protocol and a case sheet containing general information, format for recording plaque indices at different time interval was prepared [Table/Fig-1].
Schematic representation of the clinical trial.
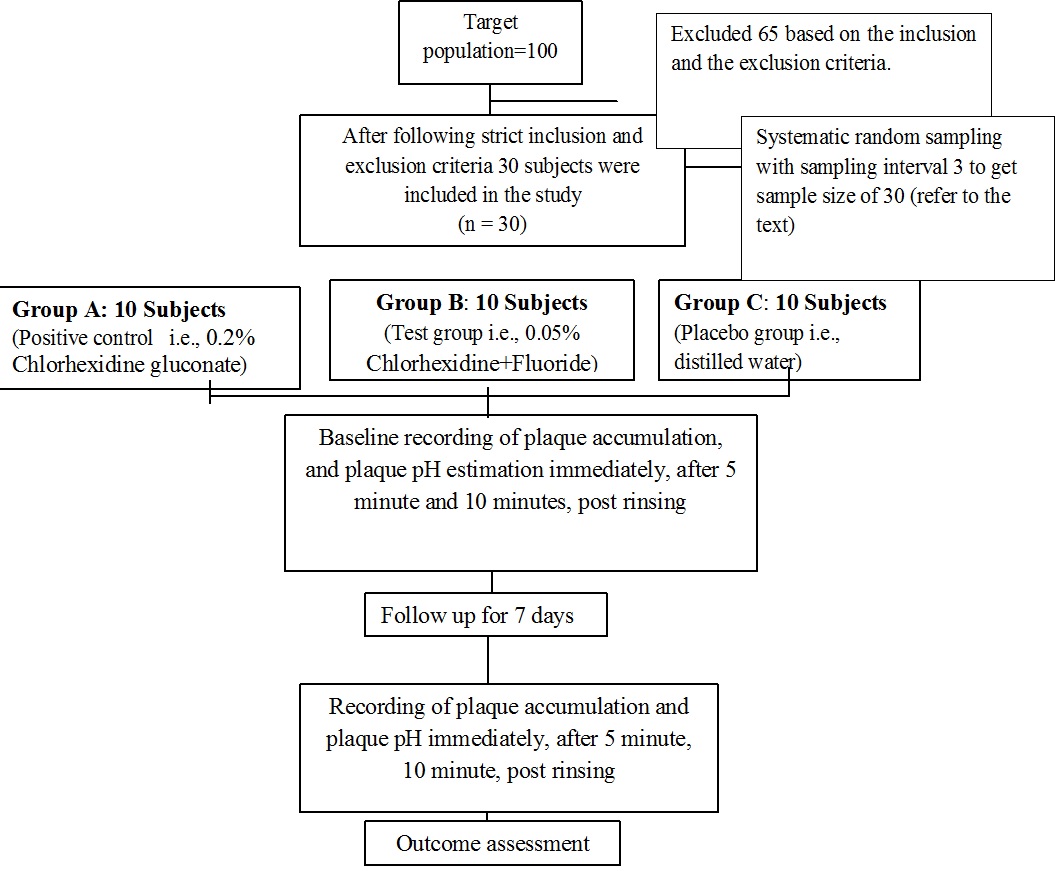
The study population consisted of 30 subjects aged 18-25 years of Sardar Patel Post Graduate Institute of Dental & Medical Sciences, Lucknow, India, attending the Out Patient Department. Initially 100 subjects were screened out of which 30 subjects were included in the study following strict inclusion and exclusion criteria.
Those subjects who gave consent, having a minimum of 20 teeth and Turesky et al., modification of Quigley Hein Plaque Index of score ≥2 for recording plaque were included in the study. Whereas subjects with history of systemic disease, who were on antibiotic therapy in past three months, allergy to test products, suffering from destructive periodontal disease, or subjects who are already using any other anti-plaque /anti-gingivitis products were excluded from the study [Table/Fig-1].
Ethical clearance was obtained from the institutional ethical committee and informed consent was taken from all the participants of the study and Clinical Trial Registry–India (CTRI) Acknowledgement no. - REF/2015/08/009511.
Randomization was done by using computer generated table of random numbers. Group allocation and distribution of the mouthwashes were performed by the third investigator. Evaluation of plaque accumulation and plaque pH was done by the first investigator who was trained and calibrated in the department with a Kappa coefficient value of 0.86 and the p-value was set at <0.05. All the mouthwashes were dispensed in identical looking plastic bottles measuring 150ml and were coded as A, B and C by the second investigator. The first investigator and the subjects were blinded about the group allocation and dispensing of the mouthwashes.
To standardize the plaque scores at baseline, thorough oral prophylaxis was performed on all the subjects before onset of the study. The subjects were asked to refrain from daily oral hygienic procedures and consumption of any food or beverages in the morning of data collection.
The subjects were recalled after a day for recording baseline scores. Baseline recording of plaque pH and scores of plaque accumulation were recorded. Based on previous literature sample size has been chosen and systematic random sampling interval 3 is the nth number which has been determined by dividing the total population size by the desired sample size [1] i.e., 30 subjects both male and female were included and randomly allocated into three groups of 10 each using lottery method:
Group A – 0.2% Chlorhexidine gluconate mouthwash (Positive control, Hexidine mouthwash manufactured by ICPA Health product Ltd.)
Group B – Chlorhexidine fluoride mouthwash [0.05% sodium fluoride] (Test group, Chlohex plusR mouthwash manufactured by Dr Reddy Laboratories Ltd.)
Group C – Distilled water (Control group or placebo).
All the subjects were instructed to rinse twice daily with 10ml of the allocated mouthwash (undiluted) for one min., after 30 minutes of brushing as interaction with anionic surfactants found within the formulations, will reduce effective delivery of Chlorhexidine in an active form. Subsequent rinsing with water was not allowed. The quantity of mouthwash given to the subjects was precalculated (10ml) at every visit.
Plaque accumulation was recorded using Turesky et al., modification of Quigley Hein Plaque Index (1970) [8]. Scores were recorded from the buccal and lingual surfaces of all fully erupted teeth after staining plaque with disclosing tablets at baseline and after one week (It has already been mentioned that the subjects were examined only twice i.e., at baseline and after seven days and same has been done for the measuring plaque pH).
Fosdick et al., (1941) method of plaque sampling and plaque measurement was followed [9]. A pooled sample of plaque was collected each time from buccal and lingual surfaces from selected teeth i.e., 16, 22, 36 and 42 with a blunt probe within 30 to 60 seconds per collection. This served as a baseline data. Plaque was then immediately suspended in 1ml of distilled water in a sterile bottle and pH was measured by the digital pH meter (pHepR pH meter, Hanna Instruments R10285) that was calibrated with distilled water to reach pH 7 every time before each sample was measured.
The subjects were asked to rinse with 10ml of test products for one minute and swish it carefully around the teeth before spitting. Post rinsing plaque samples were collected immediately, 5 and 10 minutes and the pH was estimated at baseline and after one week. The results were analysed using the SPSS version 17.0. ANOVA and post hoc Tukey’s test was employed for intra-group and inter-group comparison of plaque accumulation and plaque pH respectively.
Results
[Table/Fig-2] shows the comparison of mean plaque scores. In groups A and B there was statistically significant reduction in mean plaque scores between baseline and one week (Group A; 1.61 vs. 1.35 and Group B; 1.52 vs. 1.29).
Comparison of three groups (A, B, C) with respect to Turesky et al., modification of Quigley Hein Plaque Index scores at baseline and after one week using ANOVA.
| Groups | Baseline | 7 days |
|---|
| Mean | SD | Mean | SD | % reduction | p value |
|---|
| Group A | 1.61 | 0.10 | 1.35 | 0.13 | 16.77% | 0.0243* |
| Group B | 1.52 | 0.21 | 1.29 | 0.10 | 11.84% | 0.0076* |
| Group C | 1.51 | 0.21 | 1.42 | 0.10 | 5.96% | 0.2063 |
*p<0.05, * Statistically significant
[Table/Fig-3a,3b] Shows intra-group and inter-group comparison of plaque pH scores at baseline. In Groups A and B there was a statistically significant reduction in plaque pH scores between 0min, 5min and 10 min post rinsing with the test products (GroupA; 6.72 vs. 6.5 vs. 6.6) and (Group B; 6.74 vs. 6.68 vs. 6.7) was observed whereas intergroup comparison shows significant reduction between Group A vs. Group C from baseline to 5 min (p= 0.0588) and in Group B vs. C from baseline to 0 minute it was around p=0.0173.
Intra-group comparison of plaque pH scores at baseline using ANOVA.
| Groups | Baseline | After rinsing with mouthwashes |
|---|
| 0 min | 5 min | 10 min |
|---|
| Mean | SD | Mean | SD | % reduction p value | Mean | SD | % reduction p value | Mean | SD | % reduction, p-value |
|---|
| Group A | 6.83 | 0.14 | 6.72 | 0.27 | 0.15%, p=0.7598 | 6.5 | 0.29 | -2.64%, p=0.010* | 6.6 | 0.22 | -2.05%, p=0.025* |
| Group B | 6.79 | 0.14 | 6.74 | 0.16 | -0.29%, p=0.078* | 6.68 | 0.16 | -0.59%, p=0.077 | 6.7 | 0.29 | -0.59%, p=0.038* |
| Group C | 6.90 | 0.15 | 6.88 | 0.20 | 1.74%, p=0.1518 | 6.86 | 0.22 | 1.74%, p=0.1688 | 6.91 | 0.31 | 1.30%, p=0.5940 |
*p<0.05, statistically significant*
Inter-group comparison with respect to plaque pH at baseline using Post hoc test.
| Groups | Baseline | Changes from baseline to |
|---|
| 0 min | 5 min | 10 min | 0 min | 5 min | 10 min |
|---|
| Group A vs. Group B | p=0.6776 | p=0.9097 | p=0.1124 | p=0.4057 | p=0.5967 | p=0.0588 | p=0.3644 |
| Group A vs. Group C | p=0.4274 | p=0.8501 | p=0.0757 | p=0.3075 | p=0.0697 | p=0.0588* | p=0.2568 |
| Group B vs. Group C | p=0.2899 | p=0.5205 | p=0.7055 | p=0.8501 | p=0.117 | p=0.0173 | p=0.7337 |
*p<0.05 statistically significant*
[Table/Fig-4a,4b] Shows intra-group and inter-group comparison of plaque pH scores after one week. In Groups A and B there was a statistically significant reduction in plaque pH scores between 0 min, 5 min and 10 min post rinsing with the test products (Group A; 6.4 vs. 6.38 vs. 6.42) and (Group B; 6.56 vs. 6.51 vs. 6.54). There was also minimum drop in pre-rinsing plaque pH score in Groups A and B whereas intergroup comparison of plaque pH after one week shows no statistically significant reduction in plaque pH scores after one week between 0 min, 5 min and 10 min post rinsing with the test products.
Intra-group comparison of plaque pH scores after one week using ANOVA test.
| Groups | At 7 days |
|---|
| Baseline | After rinsing with mouthwashes |
|---|
| 0 min | 5 min | 10 min |
|---|
| Mean | SD | Mean | SD | % reduction p value | Mean | SD | % reduction p value | Mean | SD | % reduction, p-value |
|---|
| Group A | 6.76 | 0.25 | 6.4 | 0.11 | -0.15%, p=0.8785 | 6.38 | 0.12 | -2.62%, p=0.016* | 6.42 | 0.17 | -2.22%, p=0.0528* |
| Group B | 6.87 | 0.25 | 6.56 | 0.16 | 0.58%, p=0.5536 | 6.51 | 0.11 | -1.46%, p=0.1235 | 6.54 | 0.14 | -0.15%, p=0.057* |
| Group C | 6.77 | 0.23 | 6.75 | 0.15 | 0.30%, p=0.4446 | 6.84 | 0.18 | -1.03%, p=0.128 | 6.90 | 0.21 | -1.92%, p=0.5754 |
*p<0.05, statistically significant*
Inter-group comparison of plaque pH scores after one week using Post hoc test.
| Groups | Baseline | Changes from baseline to 1 week |
|---|
| 0 min | 5 min | 10 min | 0 min | 5 min | 10 min |
|---|
| Group A vs. Group B | p=0.5967 | p=0.2899 | p=0.1988 | p=0.7055 | p=0.5454 | p=0.8501 | p=0.1509 |
| Group A vs. Group C | p=0.9397 | p=0.9397 | p=0.4727 | p=0.7624 | p=0.8501 | p=0.5454 | p=0.5967 |
| Group B vs. Group C | p=0.7337 | p=0.2568 | p=0.1620 | p=0.5205 | p=0.6776 | p=0.8798 | p=0.2730 |
*p<0.05, statistically significant*
Discussion
Mouthwashes have been used from many years as it has many medicinal benefits and now-a-days its importance is gaining attention in the market as this might be a reason for the course of action of its active ingredients that has been a concern of study research and for a scientific trials [10].
The age selected for the study population is 18-25 years as the prevalence of gingivitis and periodontal disease is high from young age [11]. Around 100% of people aged 17 to 22 have gingivitis in different degrees as the rate of occurrence is high in all population [11].
Human dental plaque is one of the ecosystems in which maximum numbers of microorganisms are observed. Though a wide array of anti-plaque agents are available in the market [12], we have chosen pure composition, one brand with chlorhexidine (Mouthwash Hexidine® i.e., 0.2% chlorhexidine gluconate) and another with sodium fluoride (Clohex Plus® i.e., 0.2% chlorhexidine gluconate and 0.05% sodium fluoride) to see if there is any conjugated and synergistic effect in inhibiting plaque.
The daily supplement use of antibacterial mouthrinse in maintaining oral hygiene measures is important in inhibiting plaque formation. The cationic antiseptic chlorhexidine has often been used as a positive control during the assessment of other agents potential on plaque accumulation. However, the side effects limit its duration of use. Recently, chlorhexidine +fluoride mouthrinse formulations have become a current issue promising better tolerance and similar efficacy [12].
Researchers have suggested that fluoride enters into the plaque directly or indirectly. The retention of fluoride in the mouth after application of dental products such as dentifrices and mouthrinses may be associated with an oral fluoride reservoir. As the reservoir may serve as storage for fluoride, which releases its contents into saliva gradually and fluoride that is present in the mouth in a labile form is likely to be the most beneficial. Therefore, both fluoride and chlorhexidine containing mouthrinses have come into the market as they inhibit dental caries and plaque [13].
Till date chlorhexidine is proven to be most effective anti-plaque agent as its efficacy as a mouthrinse to inhibit dental plaque is well documented [11]. But its prolonged use is limited due to local side-effects including extrinsic tooth and tongue brown staining, taste disturbance, enhanced supragingival calculus formation and desquamation of the oral mucosa [2]. On the other hand chlorhexidine fluoride mouthwash has evidence of benefits related to reducing caries increment and reported with no side-effects as well as it can serve as a good alternative to patients who wish to avoid alcohol base (e.g. Xerostomics), sugar (e.g. Diabetics) [2]. [Table/Fig-5] shows a comparison of the results of the present study with those other similar studies in literature.
Comparison of the present study results with other similar studies in literature.
| Present study | Similar study |
|---|
| 1) In the present study the improvement in plaque scores between baseline to one week was seen in Group A and B | 1a) Study conducted by Segreto et al., in 1986, showed that chlorhexidine/fluoride rinse has equivalent activity to a 0.2% chlorhexidine gluconate formulation [7]. Anti-plaque effects of 0.05% chlorhexidine fluoride mouthrinse was similar to that of 0.2% chlorhexidine mouthrinse and significantly better than rinsing with distilled water in comparison of mean plaque score. |
| 1b) Jayaprakash et al., in 2007 demonstrated that the use of mouthrinse with the chlorhexidine-fluoride combination mouthrinse is helpful in reducing plaque and gingival index scores, at the end of the six months [13]. |
| 2) With respect to plaque pH the values recorded during baseline and one week showed a significant drop in plaque pH after 0 min, 5 min and 10 min. | 2) A minimum drop in plaque pH was also recorded after one week in pre-rinsing scores which may be attributed to the fact that continuous use of the chlorhexidine containing mouthrinse for 7 days may be postulated to have a possibility of inducing dental hypersensitivity and erosive effect on dental hard tissues with long term use. Therefore, this aspect should be investigated with long term study. |
The present study however differed from the original study model (model representing the reference consulted as a main or key article) in that the subjects instead of restraining oneself from oral hygiene measure; the mouthwash was used as an adjunct or supplement to tooth brushing [1]. Such a study design assesses the actual effectiveness of the mouthwash in a real life situation. Also a longer period study could throw light on the long term advantages and disadvantages of the chlorhexidine fluoride mouthrinse. The findings of the current study can be applied to other clinical settings and public health programmes and present study can be generalized by taking larger sample size and with longer follow-up period.
Conclusion
In preventing plaque accumulation, both Chlorhexidine and Chlorhexidine with Fluoride mouthrinses can be used as a supplement to mechanical plaque control. But Chlorhexidine with Fluoride proved to be more effective than Chlorhexidine alone as it is well accepted by the participants. So it can be used as an alternative to chlorhexidine mouthrinse.
Recommendation
Further research to establish the level of substantivity, plaque inhibition, safety and microbial parameters is necessary before this product finds a place among the other agents for daily plaque control.
*p<0.05, * Statistically significant
*p<0.05, statistically significant*
*p<0.05 statistically significant*
*p<0.05, statistically significant*
*p<0.05, statistically significant*