Anatomic Variant of Liver, Gall Bladder and Inferior Vena Cava
Yogesh Ashok Sontakke1, V. Gladwin2, Parkash Chand3
1 Assistant Professor, Department of Anatomy, Academic Centre, JIPMER, Puducherry, India.
2 Assistant Professor, Department of Anatomy, Academic Centre, JIPMER, Puducherry, India.
3 Professor and Head, Department of Anatomy, Academic Centre, JIPMER, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Yogesh Ashok Sontakke, Assistant Professor, Department of Anatomy, JIPMER Academic Centre, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry-605006, India.
E-mail: dryogeshas@rediffmail.com
The morphology and relations of liver, gall bladder and inferior vena cava are cardinal. Their anatomical variations may be a reason for the adverse surgical outcome. During routine anatomy dissection of an abdomen, we noticed a variant liver, gall bladder and inferior vena cava in a 63-year-old male cadaver. In the specimen, a retrohepatic segment of inferior vena cava was found to be intrahepatic. On dissection, it was observed that inferior vena cava was covered entirely by a liver tissue on its dorsal aspect. In the same specimen, the gall bladder had undulated inferior surface. On dissection of the gall bladder, numerous mucosal folds were present in the interior. A band of fibrous tissue was found, which was extending from the right side of the gall bladder to the falciform ligament. Hence, preoperative scanning of congenital variations of the liver, gall bladder and inferior vena cava may be compassionate in planning safe surgeries and interventional abdominal procedures.
Anatomic variations, Anatomy dissection, Intrahepatic inferior vena cava
Case Report
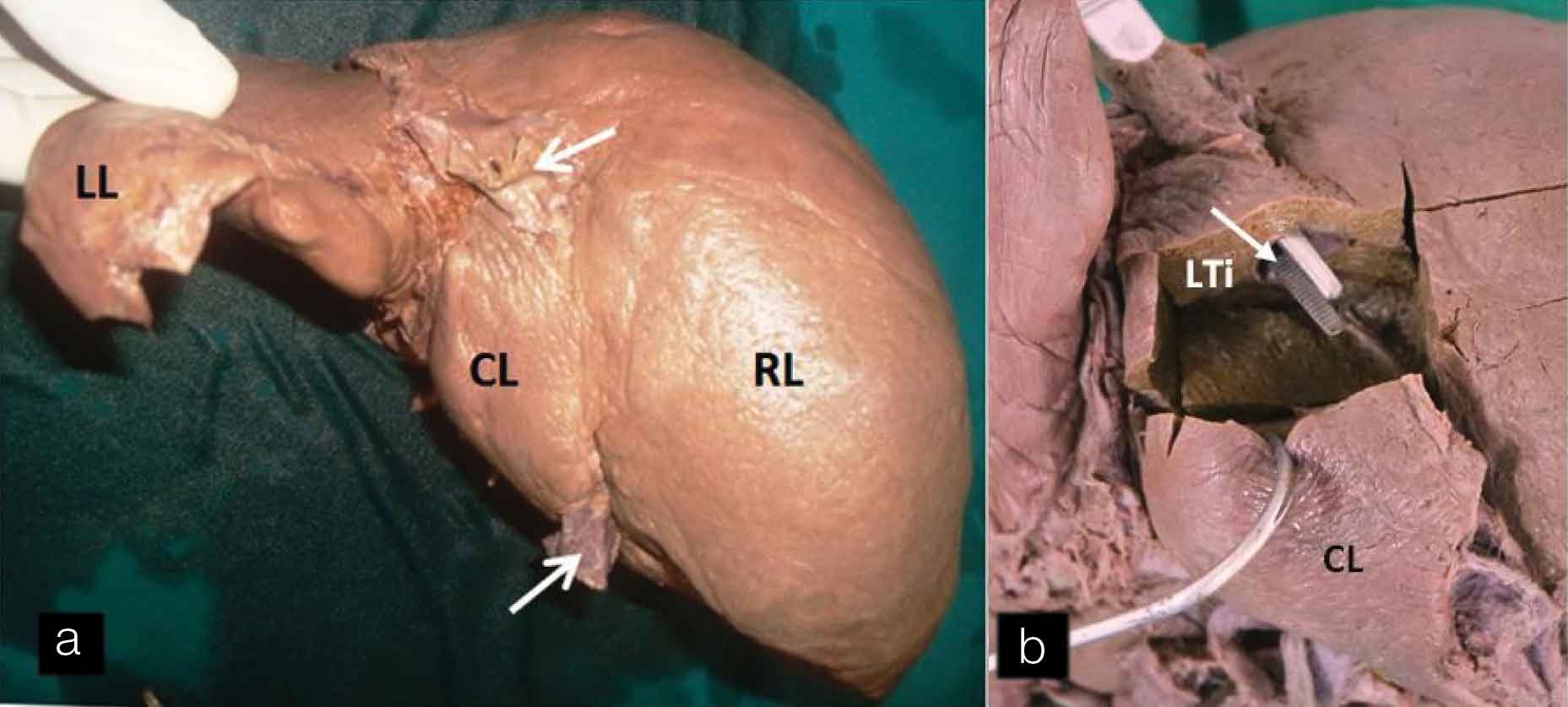
During routine anatomy dissection, we noticed a variant liver, gall bladder, and Inferior Vena Cava (IVC) in a 63-year-old male cadaver. The anterior andominal wall was dissected and the liver was taken out. On the posterior aspect of the liver, IVC was found to be passing through the substance of the liver. After removal of the liver tissue, it was observed that a retrohepatic segment of inferior vena cava was intrahepatic [Table/Fig-1a,b]. The caudate lobe was demarcated from the right lobe of liver by a shallow vertical groove present at the level of usual IVC. The peritoneum of the inferior layer of the coronary ligament was reflected from caudate lobe of the liver to the posterior abdominal wall. It was not influenced by the presence of intrahepatic IVC. On dissection of liver tissue, it was found that inferior vena cava was covered entirely by a liver tissue on its dorsal aspect. The parenchyma of the caudate lobe of the liver was found to be continuous with that of the right lobe. On tracing cranially, IVC coursed through the thoraco-abdominal diaphragm to open into right atrial chamber.
(a) Intrahepatic Inferior vena cava a. Posterior view of liver showing intra-hepatic Inferior vena cava; (b) After removal of part of liver tissue showing intrahepatic Inferior vena cava (white arrow) covered posteriorly with liver tissue (LTi). [White arrow: Inferior vena cava, CL: Caudate lobe, RL: Right lobe, LL: Left lobe].
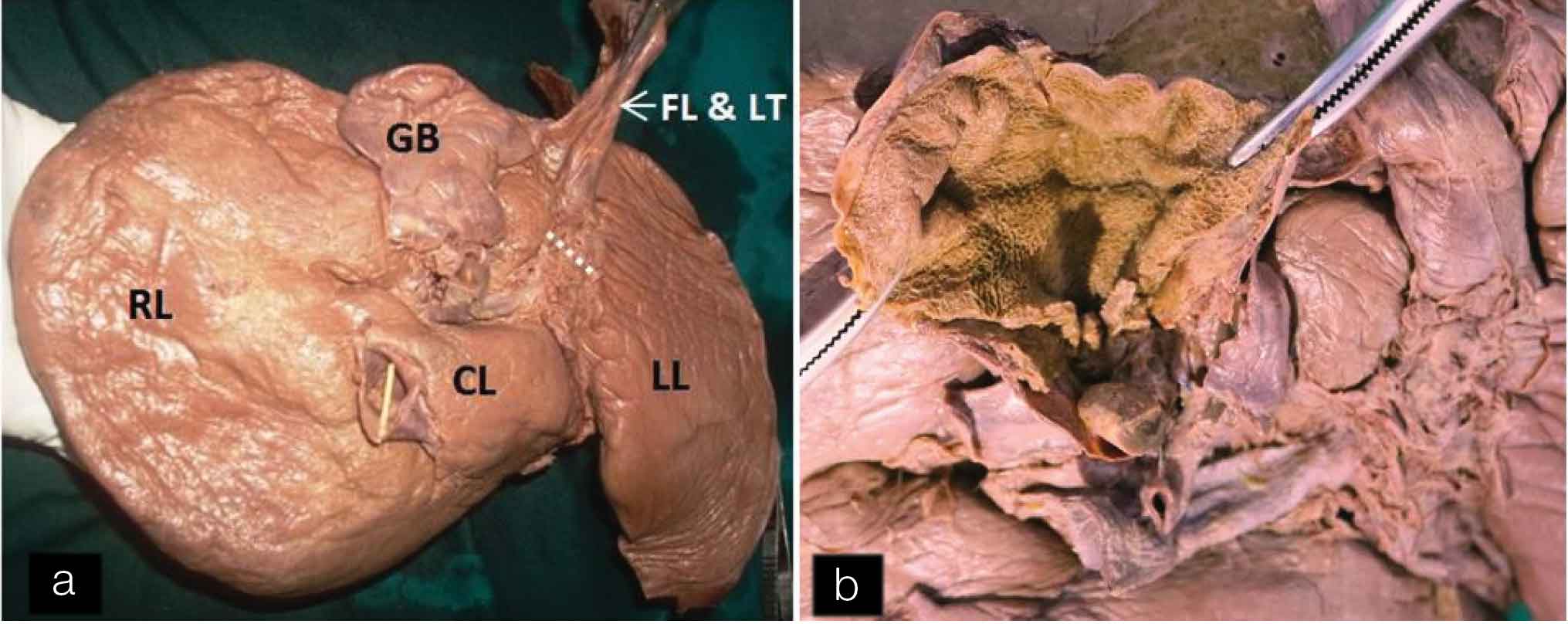
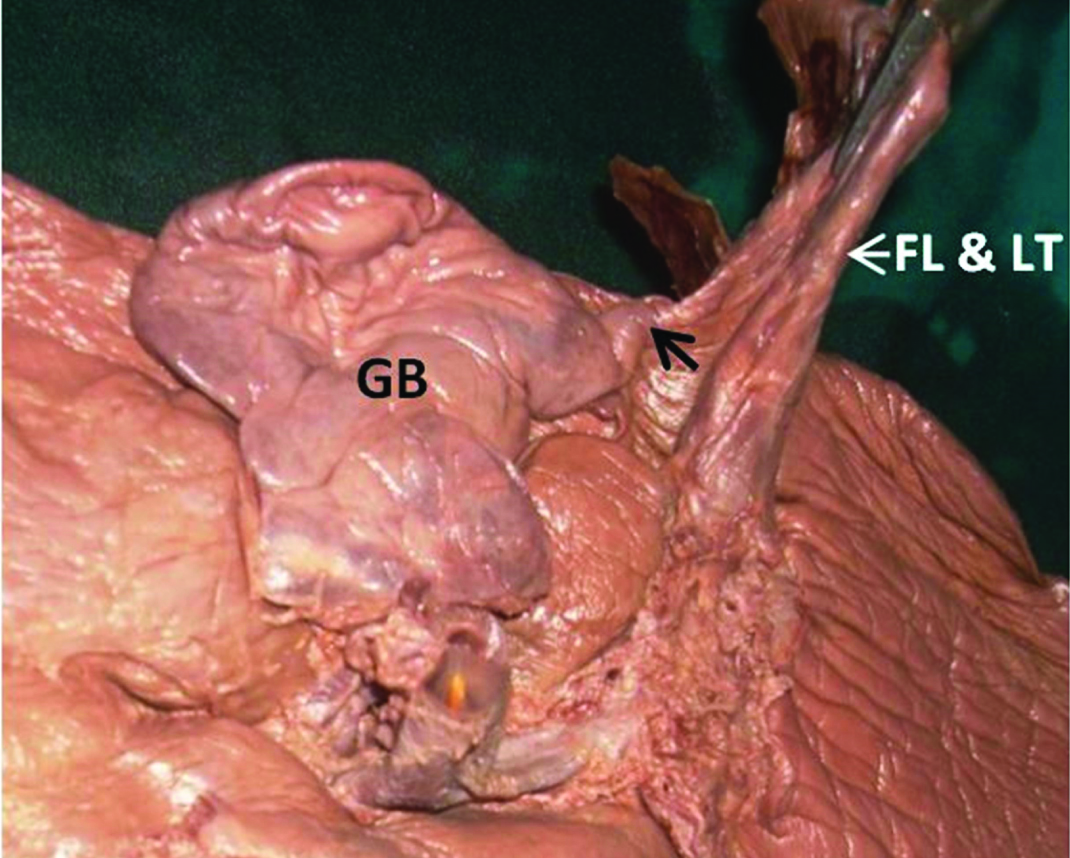
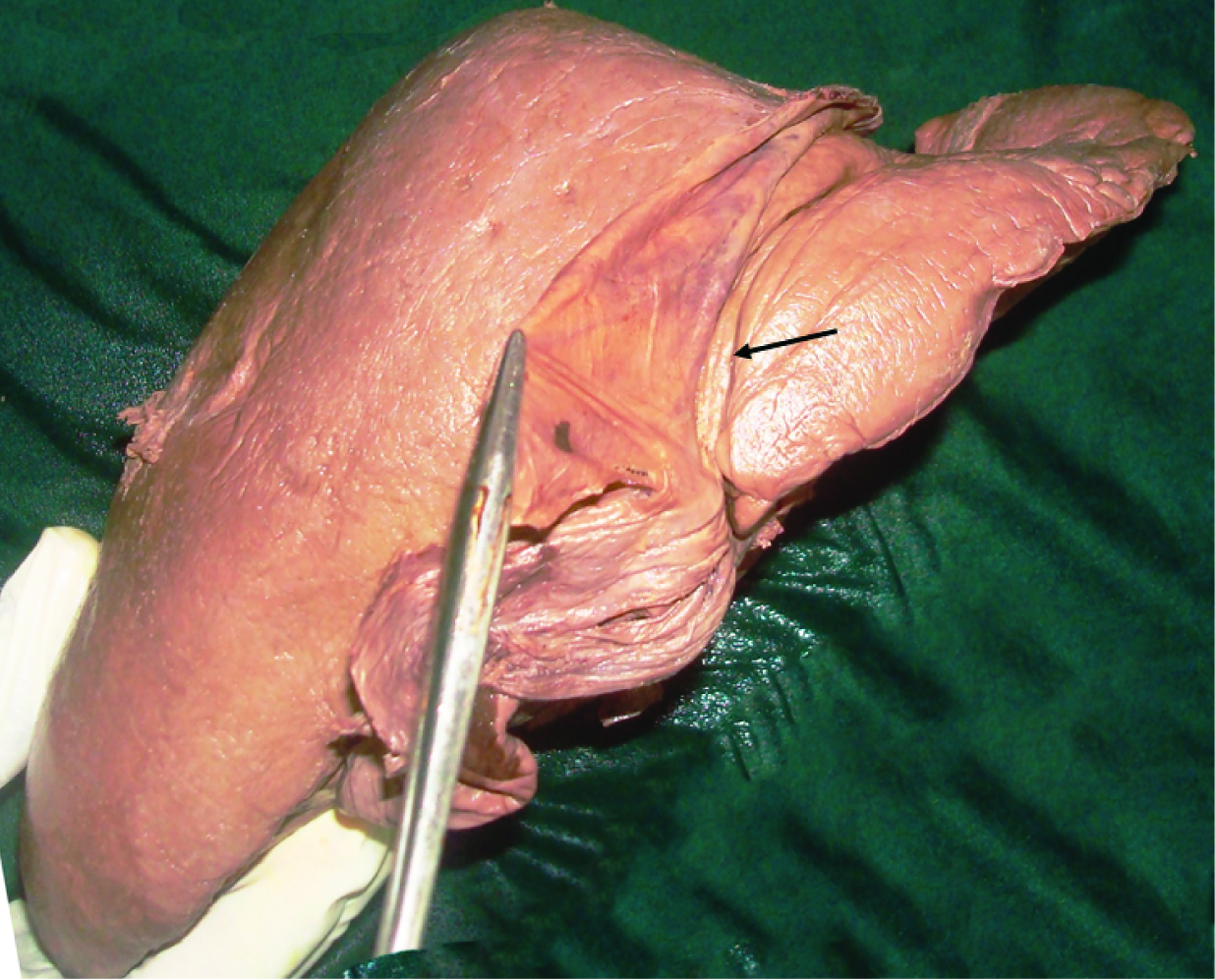
In this specimen, undulated inferior surface of the gall bladder was also seen. The inferior surface of the gall bladder was completely covered by the peritoneum. On dissection, numerous mucosal folds were seen in the interior of the gall bladder [Table/Fig-2a,b]. A band of fibrous tissue was extending from the left side of the gall bladder to the falciform ligament [Table/Fig-3]. This band was also covered on inferior aspect by peritoneum which was continuous with the peritoneum of the falciform ligament. A groove for ligamentum teres was broad and shallow. On the anterior surface of the liver, vertical fissure on left of the attachment of falciform ligament was seen [Table/Fig-4].
(a) Inferior view of liver and Gall bladder. Undulated inferior surface of gall bladder. A band of fibrous tissue extending from left side of Gall bladder (GB) to falciform ligament. A wide and shallow groove (dotted line) for ligamentum teres. (b) Interior of the gall bladder showing mucosal folds. [FL: falciform ligament, GB: Gall bladder, LT: Ligamentum teres]
Fibrous band (black arrow) extending from gall bladder to falciform ligament.
Fissure (black arrow) on anterior surface of liver, left to attachment of falciform ligament.
Discussion
The liver, largest abdominal viscera, occupies a considerable volume in the upper abdominal cavity. Usually, the ‘bare area’ of the liver is bounded medially by the IVC. The dorsal surface of the liver is formed by the caudate lobe on the left of the caval groove. In the middle of the inferior surface of the liver, there is a sharp fissure for the ligamentum teres (an obliterated fetal left umbilical vein) [1]. Earlier in the literature, many developmental anomalies of inferior vena cava have been reported including left-sided IVC double inferior vena cava, intrahepatic course of IVC, etc., [2–4].
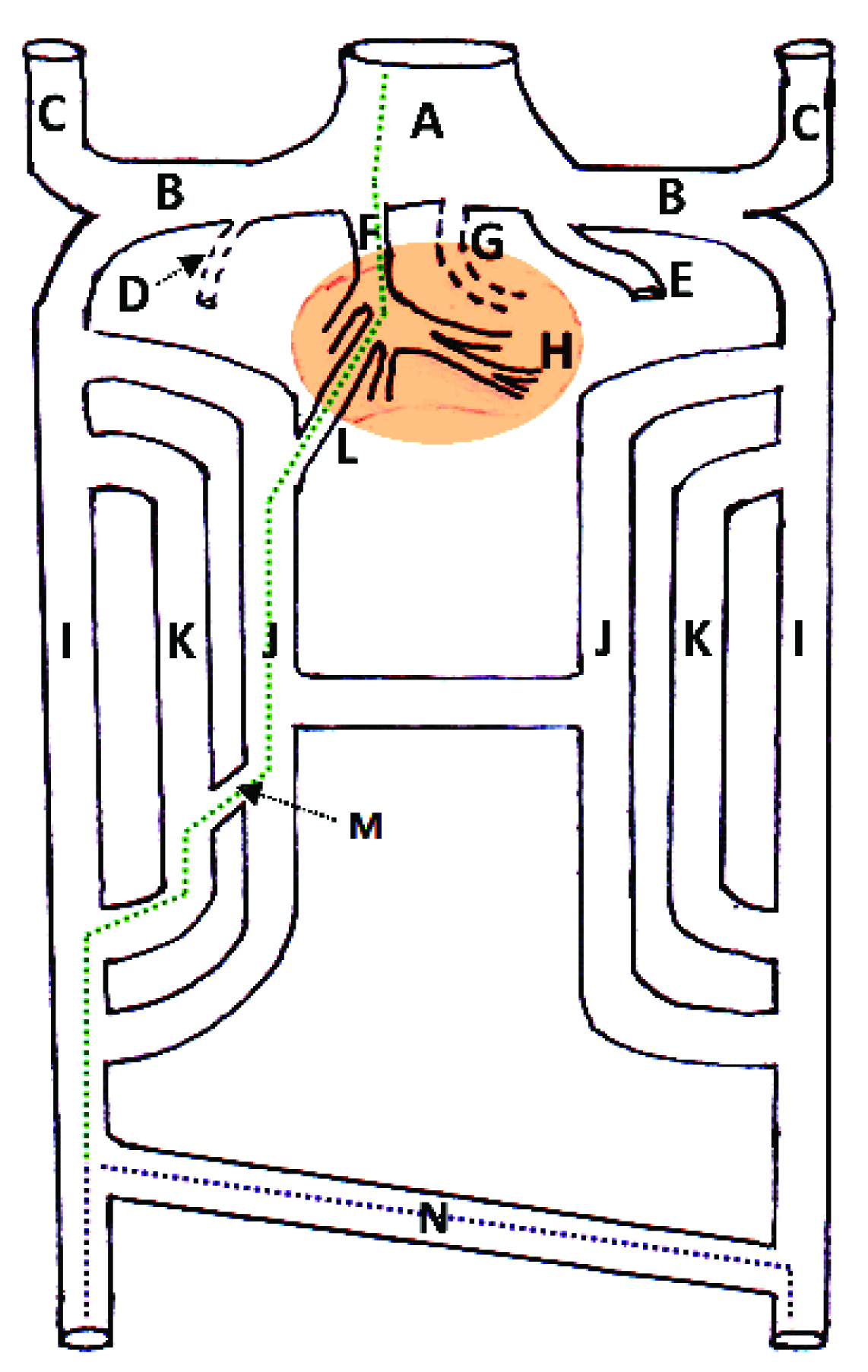
The reason for presence of intrahepatic IVC in the present case may lie in its embryological aspect. Developmentally IVC is composed of hepatic, suprarenal, renal and infrarenal segments [Table/Fig-5]. The right vitelline vein forms the hepatocardiac channel and hepatic veins. One of the developing hepatic veins, termed by the Jin et al., as posterocaudal vein, grows caudally to reach the posterocaudal surface of the liver. The hepatic segment of the inferior vena cava develops from this growing hepatic vein or posterocaudal vein which grows to merge with the subcardinal veins (Berry’s hypotheis) [5]. Deviant development of this segment may cause anomalies of a hepatic segment of IVC [2]. This developmental deviation may be a result of transformed developmental liaison between the right subcardinal vein and the right vitelline vein. This morphological variant increases potential jeopardy of traumatic injuries to the IVC during abdominal surgeries. The pre-surgical scanning of vascular anomalies reduces the frequency of complication in abdominal vascular interventions [3,4]. These pre-surgical scans for congenital variation of IVC may be helpful in planning safe abdominal surgeries as well as interventional or diagnostic procedures such as IVC filter placement, renal venous sampling, etc., [6]. The maximum intensity projection contrast enhanced computed tomography imaging as an imperative tool for a non-invasive method of diagnosing morphological IVC variations, and it will be helpful in describing IVC relationship with adjacent viceras [6].
Schematic diagram showing development of the inferior vena cava. [Green dotted line: Developing inferior vena cava, Dotted purple line: developing common iliac veins, A: Sinus venous, B: Common cardinal vein, C: Anterior cardinal veins, D: degenerating right umbilical vein, E: Left umbilical vein, F: hepato-cardiac channel developing from right vitelline vein, G: degenerating left vitelline vein, H: developing liver in septum transversum (Brown area), I: Posterior cardiac veins, J: Subcardinal veins, K: Supracardianl veins, L: Subcardinal-hepatic anastomosis, M: Supracardinal-subcardinal anastomosis, N: Posterior cardinal venous anastomosis].
The gall bladder is a pear-shaped diverticulum. Cystic duct con-nects the gall bladder with the common bile duct. The gall bladder is adhered to the inferior surface of the liver. Its inferior surface is covered by a layer of the peritoneum. The quadrate lobe of the liver lies between the fissure for the ligamentum teres and the gallbladder [1]. In view of clinical aspects, knowledge of the usual and variant anatomy of extrahepatic biliary tree (EHBT) is important in abdominal surgeries [7]. An incidence of abnormal anatomical variations of Extra-Hepatic Biliary tract was 30-47% [8]. These variations include accessory hepatic ducts, accessory cystico-hepatic ducts, low insertion of the cystic duct. Rabinovitch et al., reported two autopsy cases of the bilobed gall bladder in his study and termed it as a rare anomaly. These variations of biliary tract were seen to be associated with high incidence of biliary tract injuries in abdominal surgeries [8].
The reason behind the undulated inferior surface of gall bladder is not earlier described in literature. The undulated surface may be traced to the developmental stage through which this structure passes [8]. In the present case, the gall bladder might have developed as multiple outgrowing pouches from cystic bud. Initially the cavities of outgrowing pouches might have separated from each other by septae. In later stage of the development, all the cavities might have been fused internally to form single cavity with numerous prominent mucosal folds. On the external surface the incomplete resolution or non-uniform fusion of the pouches might have produced undulation. One of the outgrowing pouches of the developing gall bladder might be extending upto the falciform ligament initially but due to resolution and further development, its cavity became small, but that attachment remains as fibrous band extending from gall bladder to falci from ligament. There is need of further embryological study in future to find reason for the undulated nature of the gall bladder. The non-uniform interior of the gall bladder is the reason for reduced motility of the viscera. This may produce stagnation of the bile and may also be significantly associated with recurrent abdominal colic, with jaundice, or with stone formation due to stagnation of the bile and also acute cholecystitis [9]. Ultrasound evaluation, computed tomography, magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiography (ERCP) may assist in the diagnosis of the condition [9].
The developing liver parenchyma is grooved by the left umbilical vein which passes through deep sulcus. Later on, the sulcus deepens and the left umbilical vein gets obliterated to form a ligamentum teres. This ligament is covered by a fold of peritoneum and it extends in falciform ligament upto the umbilicus. The widened fissure for ligamentum teres may be indicative of the failure of complete development of the liver parenchyma resulting into its non-approaching right lobe and quadrate lobe. The wide fissure for ligamentum teres as noticed in this study report may create confusion in the mind of the radiologist regarding the usual visualisation of the fissure and dimensions of the right and the left lobes may be mistaken [10].
In the present case we reported the groove to the left of the falciform ligament i.e., on the diaphragmatic surface of the liver (anterosuperior surface). Joshi et al., also reported prominent vertical grooves on the anterosuperior surface, i.e., diaphragmatic sulci in 6% of the livers [10]. The diaphragmatic sulci may be a consequence of the uneven development of the hepatic parenchyma and may be caused by the variable resistance of different muscle bundles of the diaphragm [11]. These fissures may also be attributed to the existence of weak zones of hepatic parenchyma as the weak zones offer a lower resistance to external pressure of the diaphragm. These sulci could represent a useful guide for surface projection of the portal fissures and the hepatic veins running through them [11].
Conclusion
The morphology and relations of the liver and gall bladder are fundamental. Anatomical variations of the liver and gall bladder may be a reason of exacerbating surgical outcome. Preoperative scanning of congenital variations of the liver and gall bladder may be helpful in planning safe abdominal surgeries as well as interventional or diagnostic abdominal procedures.
[1]. Standring S, Gray’s anatomy: anatomical basis of clinical practice 2008 40th editionChurchillLivingstone Elsevier:1163-80. [Google Scholar]
[2]. Chuang V, Mena C, Hoskins P, Congenital anomalies of the inferior vena cava. Review of embryogenesis and presentation of a simplified classificationThe British Journal of Radiology 1974 47(556):206-13. [Google Scholar]
[3]. Srivastava A, Singh K, Suri A, Vijjan V, Dubey D, Inferior vena cava in urology: importance of developmental abnormalities in clinical practiceThe Scientific World Journal 2005 5:558-63. [Google Scholar]
[4]. Jimenez Gil R, Morant Gimeno F, Major venous anomalies and abdominal aortic surgeryInteractive CardioVascular and Thoracic Surgery 2010 10(4):631-33. [Google Scholar]
[5]. Jin Z, Cho B, Murakami G, Fujimiya M, Kimura W, Yu H, Fetal development of the retrohepatic inferior vena cava and accessory hepatic veins: Re-evaluation of the Alexander Barry’s hypothesisClin Anat 2010 23:297-303. [Google Scholar]
[6]. Abdullah A, Williamson K, Lewis T, Elsamaloty H, Variant ventral intrahepatic course of inferior vena cava: volume-rendering and maximum intensity projection CT findingsThe British Journal of Radiology 2011 84(1003):e135-37. [Google Scholar]
[7]. Khayat Abnormal anatomical variations of extra-hepatic biliary tract, and their relation to biliary tract injuries and stones formationGastroenterology Research 2014 7:12-16. [Google Scholar]
[8]. Rabinovitch J, Rabinovitch P, Rosenblatt P, Pines B, Congenital anomalies of the gallbladderAnnals of Surgery 1958 148(2):161-68. [Google Scholar]
[9]. Karaca T, Yoldas O, Bilgin B, Bilgin S, Evcik E, Ozen S, Diagnosis and treatment of multiseptate gallbladder with recurrent abdominal painCase Reports in Medicine 2011 2011:1-2. [Google Scholar]
[10]. Joshi SD, Joshi SS, Athavale SA, Some interesting observations on the surface features of the liver and their clinical implicationsSingapore Med J 2009 50(7):715-19. [Google Scholar]
[11]. Macchi V, Feltrin G, Parenti A, De Caro R, Diaphragmatic sulci and portal fissuresJournal of Anatomy 2003 202(3):303-08. [Google Scholar]