Spondyloarthritis (SpA) is an umbrella term for chronic inflammatory diseases that have involvement of joints, entheses and some extraarticular manifestations (such as acute anterior uveitis, psoriasis and inflammatory bowel diseases). An association with the HLA-B27 antigen is usually demonstrable. Based on the predominant clinical manifestation, SpA is classified into Axial (ASpA) and Peripheral (PSpA) varieties [1]. ASpA comprises of conditions affecting predominantly the spine and/or the sacroiliac joints, such as Ankylosing Spondylitis (AS), nonradiographic axial Spa, psoriatic arthritis and reactive arthritis with axial involvement, and arthritis associated with inflammatory bowel disease [2].
The 2015 ACR/ SAA/ SPARTAN (American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network) recommendations suggest the use of TNF-α (tumour necrosis factor-α) inhibitors for adult A SpA patients not responding to NSAIDs (Non-steroidal anti-inflammatory drugs) [3]. The presently approved TNF-α inhibitors include infliximab, Adalimumab, Etanercept, Golimumab, and Certolizumab pegol [2].
Though the biological response modifiers (BRMs)have been proven to be effective in patients with ASpA not responding to NSAIDs, their exorbitant cost is the major hindrance for their regular usage, especially in India where most of the treatment is borne by the patient through out-of-pocket spending [4]. Despite the arrival of biosimilars, the cost of therapy with BRMs is still comparatively higher and consequently many Asian Indian patients with ASpA are deprived of the benefits of these agents.
Ours is a specialist state-owned healthcare organization which provides BRMs for patients with ASpA and other rheumatological conditions through government procurement. We were curious to know the comparative clinical outcomes of the usage of different BRMs for patients with AS who have therapeutic failure with NSAIDs. With this background we report a physician’s experience of biologics usage in Asian Indian patients with AS.
Materials and Methods
We collected data from patients with AS attending the rheumatology outpatient department at the INHS Asvini Hospital, Mumbai, over 2 years between 1st January 2014 and 31st December 2015, referred from across the country.
AS patients who were judged to be having therapeutic failure after adequate therapy with NSAIDs for at least 6 months were put on treatment with either Etanercept (given as 50 mg per sitting, subcutaneously; dosing was once a week upto 4 months, once every 2 weeks for 4 months, and once a month for 4 months) or Infliximab (given as 5mg/kg intravenous infusion every 2nd month for 8 doses). Mean dose for Etanercept was 24 injections; the dose of Etanercept was tapered following a fixed protocol (weekly dosing for 3 months – 12 doses; fortnightly dosing for 3 months – 6 doses; and monthly dosing for 6 months - 6 doses; thus a total of 24 doses). The allocation of treatment was determined by patient convenience (local patients can conveniently visit the hospital more frequently; for them we gave ETN which has a weekly dosing pattern. For outstation patients who cannot come to the hospital frequently, we gave them Infliximab which requires one dose only every 2 months) and physician discretion (the guidelines suggest that any BRM can be used in the initial stages and does not specify which BRM to be used) as guided by the 2015 ACR/ SAA/ SPARTAN (American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network) recommendations [3]. NSAID therapy was stopped in these patients. Randomization was not performed for treatment allocation in these patients. Further, since this was not a clinical trial but a compilation of experience with different agents, informed consent waiver was obtained from the institutional ethics committee.
At the initiation of therapy, demographic details, time to diagnosis of AS, the duration of disease, presence of low backache, early morning stiffness, peripheral joint involvement, ocular, dermatological, gastrointestinal and genitourinary involvement were recorded, total joint count and peripheral joint count were noted, ESR (erythrocyte sedimentation rate), CRP (C-reactive protein)values and HLA-B27 score were obtained. Baseline values of scores of BASMI-3 (Bath AS Metrology Index) and MASES (Maastricht AS Enthesitis Score) were calculated. To monitor the disease activity, BASDAI (Bath AS Disease Activity Index) and ASDAS (AS Disease Activity Score) – ESR scores were recorded at baseline, and after 6 months and 12 months of therapy initiation.
All data was recorded electronically and analysed using SPSS v22; p<0.05 was considered to be statistically significant for all tests applied.
Results
This study included a compilation of data of a total of 35 patients with AS treated with BRMs. Out of the 35 patients, 15 patients received Etanercept, and 20 received Infliximab.
Out of the 35 patients, 3 were female (1 Etanercept, 2 Infliximab) and 32 were male (14 Etanercept, 18 Infliximab). The demographic and baseline parameters of the patients is summarised in [Table/Fig-1]. Overall, both groups were comparable in all parameters except in the baseline BASMI-3 score which was significantly higher in patients who received Etanercept.
Baseline and demographic parameters of patients with AS treated with Etanercept and Infliximab.
| Parameter (mean values) | Overall (n=35) | Etanercept group(n=15) | Infliximab group(n=20) | p-value† |
|---|
| Age (years) | 33.74±7.88 | 34.53±9.55 | 32.45±6.48 | 0.474 |
| Time to diagnosis (months) | 24.54±26.90 | 27.40±29.97 | 22.40±24.93 | 0.604 |
| Duration of disease (months) | 81.60±47.18 | 89.60±48.82 | 75.60±46.25 | 0.397 |
| Baseline BASMI-3 score | 1.77±2.10 | 2.73±2.66 | 1.05±1.19 | 0.034 |
| Baseline MASES score | 0.37±0.91 | 0.20±0.56 | 0.50±1.10 | 0.302 |
| Total Joint Count | 0.54±0.92 | 0.60±0.91 | 0.50±0.95 | 0.754 |
| Swollen Joint Count | 0.40±0.81 | 0.33±0.72 | 0.45±0.89 | 0.671 |
| ESR (mm at 1 hour) | 34.49±16.45 | 39.47±19.96 | 30.75±12.50 | 0.151 |
| Baseline BASDAI score | 4.22±1.34 | 3.91±1.42 | 4.45±1.26 | 0.252 |
| Baseline ASDAS-ESR score | 3.33±0.84 | 3.05±0.79 | 3.53±0.83 | 0.092 |
†independent samples T test.
Out of the 35 patients, 6 patients (3 Etanercept, 3 Infliximab; all males) had history of early morning stiffness. Further, 10 patients (5 Etanercept, 5 Infliximab; all males) gave history of peripheral joint involvement in the knee (5), ankle (3), shoulder (2) and elbow (1); one patient had both knee and ankle joint affliction. All patients had low backache, 2 patients had ocular involvement, one patient had a skin rash, and no patient gave history of gastrointestinal or genitourinary involvement. A total of 25 patients (11 Etanercept, 14 Infliximab) were positive for HLAB27 status, and 25 patients (5 Etanercept, 20 Infliximab) had positive values for CRP.
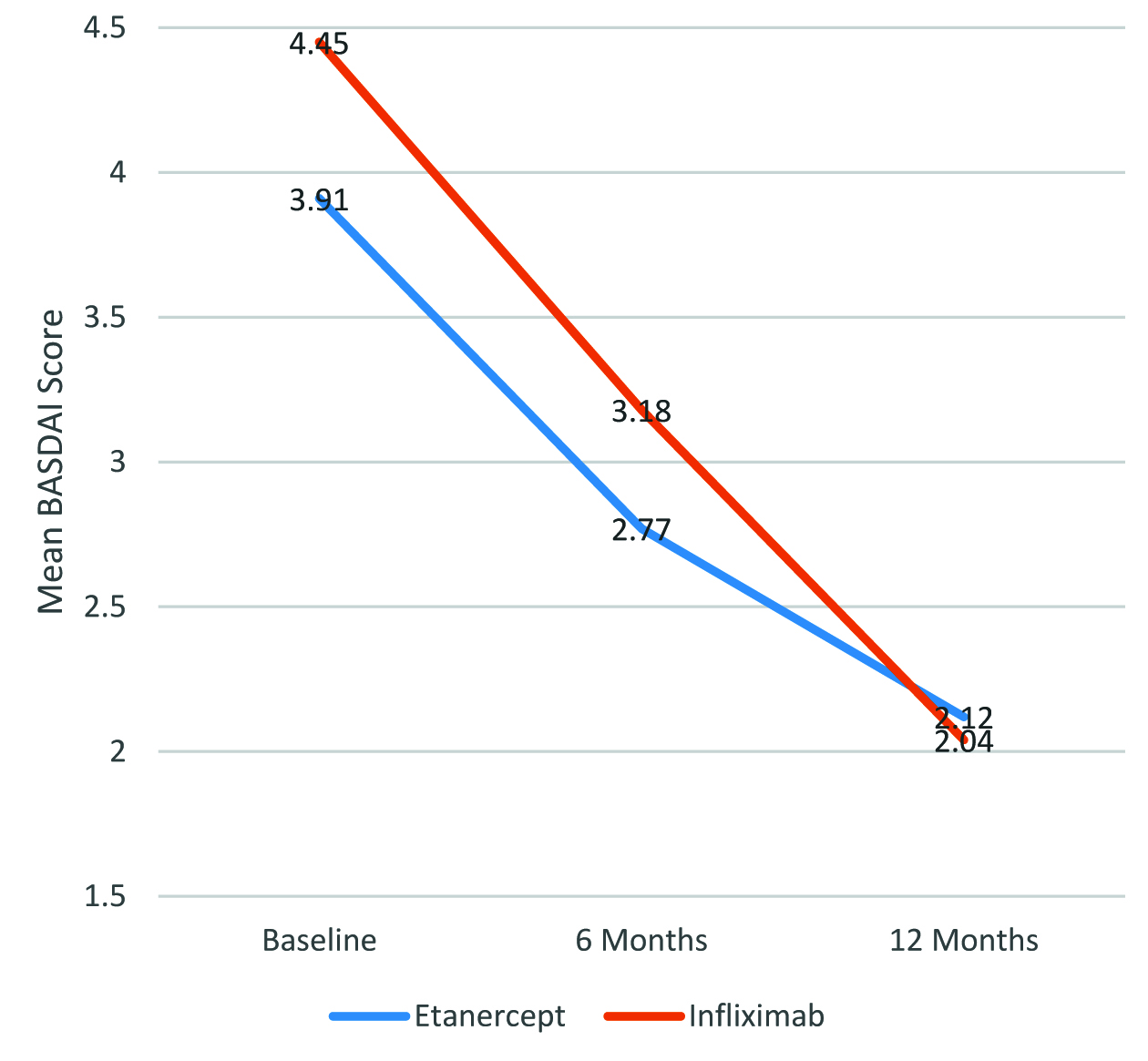
The mean BASDAI scores at baseline, 6 months, and 12 months is summarised in [Table/Fig-2]. The distribution of mean BASDAI scores over 12 months is shown in [Table/Fig-3]. Overall, the reduction in disease activity, as evidenced by reduction in the mean BASDAI scores over 12 months of treatment was statistically significant for all patients when considered together, as well as when Etanercept and Infliximab were considered separately (p<0.0001 in all cases). However, there was no statistically significant difference in the magnitude of reduction in the mean BASDAI scores between patients who received Etanercept and those who received infliximab (p=0.696).
Mean BASDAI scores over 12 months in AS patients receiving Etanercept and Infliximab.
| Overall(n=35) | Etanercept group(n=15) | Infliximab group(n=20) |
|---|
| Baseline | 4.22±1.34 | 3.91±1.42 | 4.45±1.26 |
| 6 months | 3.00±0.78 | 2.77±0.82 | 3.18±0.71 |
| 12 months | 2.07±0.41 | 2.12±0.62 | 2.04±0.13 |
| p-value† | <0.0001 | <0.0001 | <0.0001 |
| 0.696 |
†Repeated measures ANOVA
Mean BASDAI over 12 months in AS patients receiving Etanercept and Infliximab
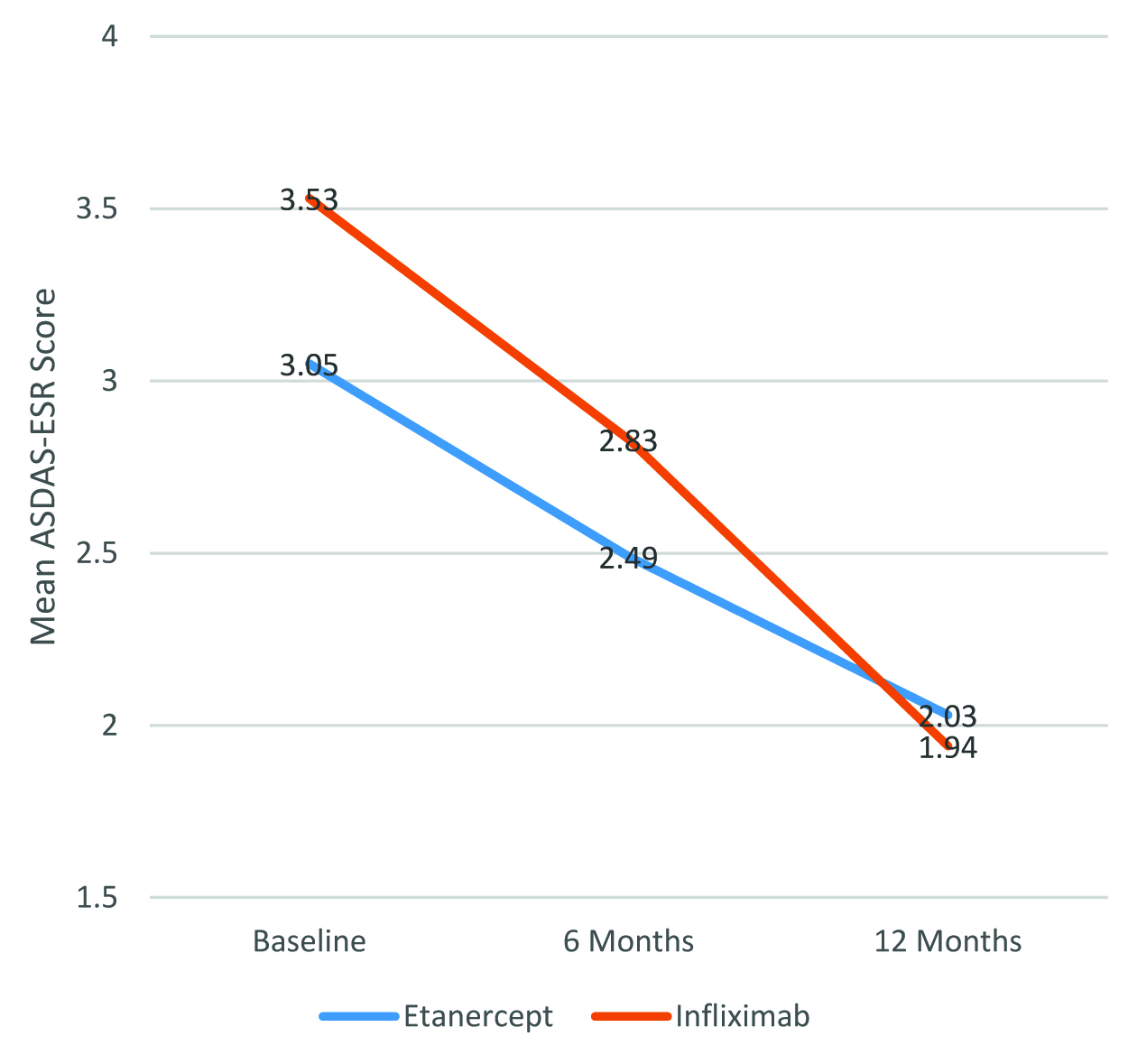
The mean ASDAS-ESR scores at baseline, 6 months, and 12 months is summarised in [Table/Fig-4]. The distribution of mean ASDAS-ESR scores over 12 months is shown in [Table/Fig-5]. Overall, the reduction in disease activity, as evidenced by reduction in the mean ASDAS-ESR scores over 12 months of treatment was statistically significant for all patients when considered together, as well as when Etanercept and Infliximab were considered separately (p<0.0001 in all cases). However, there was no statistically significant difference in the magnitude of reduction in the mean ASDAS-ESR scores between patients who received Etanercept and those who received infliximab (p=0.618).
Mean ASDAS-ESR scores over 12 months in AS patients receiving Etanercept and Infliximab.
| Overall (n=35) | Etanercept group (n=15) | Infliximab group (n=20) |
|---|
| Baseline | 3.33±0.84 | 3.05±0.79 | 3.53±0.83 |
| 6 months | 2.68±0.61 | 2.49±0.43 | 2.83±0.72 |
| 12 months | 1.98±0.39 | 2.03±0.24 | 1.94±0.46 |
| P value† | <0.0001 | <0.0001 | <0.0001 |
| 0.618 |
†Repeated measures ANOVA
Mean ASDAS-ESR over 12 months in AS patients receiving Etanercept and Infliximab
Both BRMs were well-tolerated. Two patients who received infliximab reported mild, self-limited flu-like transfusion reaction, and two other patients had mild macular self-limiting rashes. Four patients on Etanercept had transient depression, epiphora and mild macular self-limiting rashes. There were no serious ADRs which required in treatment withdrawal or any other serious consequences to any patient.
Discussion
TNF-α based BRMs represent the only reliable treatment option available at the present time for treating ASpA patients who do not respond to the first line of therapy which are the NSAIDs [2].
According to the 2015 ACR/ SAA/ SPARTAN (American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network) recommendations, the first line of therapy for A SpA involves NSAIDs with non-pharmacological treatment modalities, and in case of non-response to, intolerance to, or disease progression despite NSAIDs, TNF-α inhibitors may be initiated as second line of therapy [3]. In PSpA patients with NSAID failure, there is an option of using SAARDs (slow-acting anti-rheumatic drugs) and local corticosteroids [2] before initiating BRM therapy; however, such an option is not available for ASpA. The recommendations do not recommend any specific TNF-α inhibitors except in cases of ASpA with IBD where monoclonal antibodies are to be preferred over Etanercept [3].
In our study, we administered BRMs based on patient convenience: local patients were given weekly Etanercept, and outstation patients were administered 2nd monthly Infliximab. The other anti-TNFα BRMs (Adalimumab, Golimumab and Certolizumab pegol) were not readily available in India at the time of initiation of this study. The baseline BASMI-3 score was higher in patients who received Etanercept; despite this difference, the impact of both the drugs on disease activity over a period of 12 months was comparable and not statistically significant, as evidenced by a similar reduction in the mean BASDAI and mean ASDAS-ESR scores over a period of 12 months. To the best of our knowledge, this is the first instance where the effect of different BRMs on disease activity has been documented and reported in Asian Indian patients with AS.
Previous studies reported elsewhere concur with our observations that different biologic TNF-α inhibitors (Infliximab, Etanercept, Adalimumab, and Golimumab) demonstrate similar efficacy as measured by % of patients achieving ASAS40 response in AS patients with NSAID failure [5–8]. A 2015 meta-analysis also reports that the TNF-α inhibitors, in comparison with placebo, significantly improve disease activity and functional capacity [9]. However, our study was a direct head-to-head comparison of efficacy and safety of two BRMs in AS where we demonstrated similar efficacy and safety.
We could not reliably comment on the various factors which predict the treatment outcome to either of the BRMs in our study because of the smaller sample size. Previous studies with larger sample of 1250 patients [10] and 635 patients [11] have suggested that young age, short disease duration, low level of functional disability, elevated acute phase reactants and signs of active inflammation are associated with positive treatment response to TNF-α inhibitors.
Limitation
The major limitation of our study was its inadequate sample size; however, keeping in mind the expenditure incurred in the administration of a BRM, these results should not be discarded. Further, since randomization was not followed and allocation of drug was based on factors such as severity of disease and patient convenience, the impact of these factors on the outcome cannot be ruled out completely. Keeping in mind the results of this study, further randomized studies are warranted with a larger sample size. Finally costs benefit analysis regarding the usage of BRMs in AS would have given a complete picture of the BRM usage in the Indian set-up. Such an analysis wherein two BRMs are compared on a cost-benefit platform will provide invaluable information not only to physicians, but also to policy-makers, and insurers. Further studies should be done keeping in mind this pharmaco-economic angle as well.
Conclusion
To conclude, for Asian Indian AS patients with NSAID failure, Etanercept and Infliximab offer statistically similar reduction in disease severity, as evidenced by reduction in BASDAI and ASDAS-ESR scores over a period of 12 months. Further studies with larger sample size are required to find out the profile of patients expected to show a greater positive response to these BRMs in this population.
†independent samples T test.
†Repeated measures ANOVA
†Repeated measures ANOVA