Adjuvant radiotherapy following mastectomy for breast carcinoma offers an unequivocal benefit on local control and survival in patients with stage IIB and III disease [1,2]. Traditionally, adjuvant radiotherapy following mastectomy was delivered by conventional fractionation of 45 to 50 Gy in 1.8 to 2 Gy per fraction over a duration of five weeks. Hypofractionated Radiotherapy (HFRT) offers the convenience of shorter overall treatment time (of two to 3.5 weeks) and reduced cost in the scenario of an ever increasing evidence of equivalent survival and toxicity when compared to conventional RT [3]. As a consequence, HFRT is being increasingly adopted across centers. In this study, we report on the dose received by the Organs at Risk (OARs) and acute toxicities experienced by our patients following HFRT to the chest wall.
Materials and Methods
This prospective observational study was conducted at our center between October 2014 and June 2015. Women with diagnosed breast cancer who had undergone mastectomy and required adjuvant RT to the chest wall with or without axilla and supraclavicular lymph nodes were included. These included patients with pT3 or higher disease, and patients with node positive disease. Patients older than 70 years were excluded, as were those with loco-regionally recurrent tumours, microscopically positive margins, a history of connective tissue disorders and/or cardiac and pulmonary morbidities. Clearance was obtained from the Institutional Ethics committee prior to the conduct of the study. The patients were recruited only after they consented to participate and signed the informed consent document.
All patients were planned for adjuvant HFRT to the chest wall. In addition, patients were also planned for RT to supraclavicular fossa when there was histopathological evidence of axillary node metastases, and in scenario where neoadjuvant chemotherapy was administered prior to definitive surgery. All patients were immobilized while ‘free breathing’ using a thermoplastic mould in supine position with both arms extended above their head, abducted and externally rotated. Patients with inadequate lymph node dissection (less than 10 nodes examined pathologically) were also considered for RT to the supraclavicular fossa. Scar sites were marked using lead markers. Patients underwent CT simulation in the same position without IV contrast. The Clinical Tumour Volume (CTV) was contoured according to the Radiotherapy and Oncology Group (RTOG) guidelines [4]. The Planning Target Volume (PTV) was then generated by giving a one cm isometric margin to CTV. Organs at Risk (OAR) contoured included heart, lung and spinal cord. Lung was contoured in pulmonary window, with inclusion of all inflated and collapsed, fibrotic and emphysematous lung and small vessels extending beyond the hilar region. Hila and trachea/main bronchus were excluded from the lung volume. The heart was contoured along with the pericardial sac, beginning superiorly at the level of the inferior aspect of the pulmonary artery passing the midline, and extending inferiorly upto the apex of the heart.
All patients were planned by 3D-Conformal RT (3D-CRT) technique with megavoltage beams on a multiple energy ELEKTA Linear Accelerator, with HFRT (42.5 Gy in 2.66 Gy per fraction, one fraction per day, five days per week delivered over three weeks). The photon energy used was either 6 MV or 15 MV. Beam arrangement included medial and lateral opposed tangential fields to irradiate the chest wall, with or without the use of a single anterior field (with a gantry tilt of 5-10 degrees to avoid the spinal cord and oesophagus) for the supraclavicular region using mono isocentric technique. The treatment was planned with a goal of 100% volume of PTV to be covered by 95% isodose line [Table/Fig-1]. Dose homogeneity was optimized using wedges and ‘field-in-field’ technique using multi leaf collimators. Data collected included the volume of PTV receiving at least 95% and 90% of prescribed dose (V95 and V90) and also dose delivered to 90% of the volume of PTV (D90%) from the dose volume histogram. Similarly, mean lung dose and volume of lung receiving at least 20% (V20) of the prescribed dose and mean heart dose and volume of heart receiving at least 25% (V25) of the prescribed dose were documented.
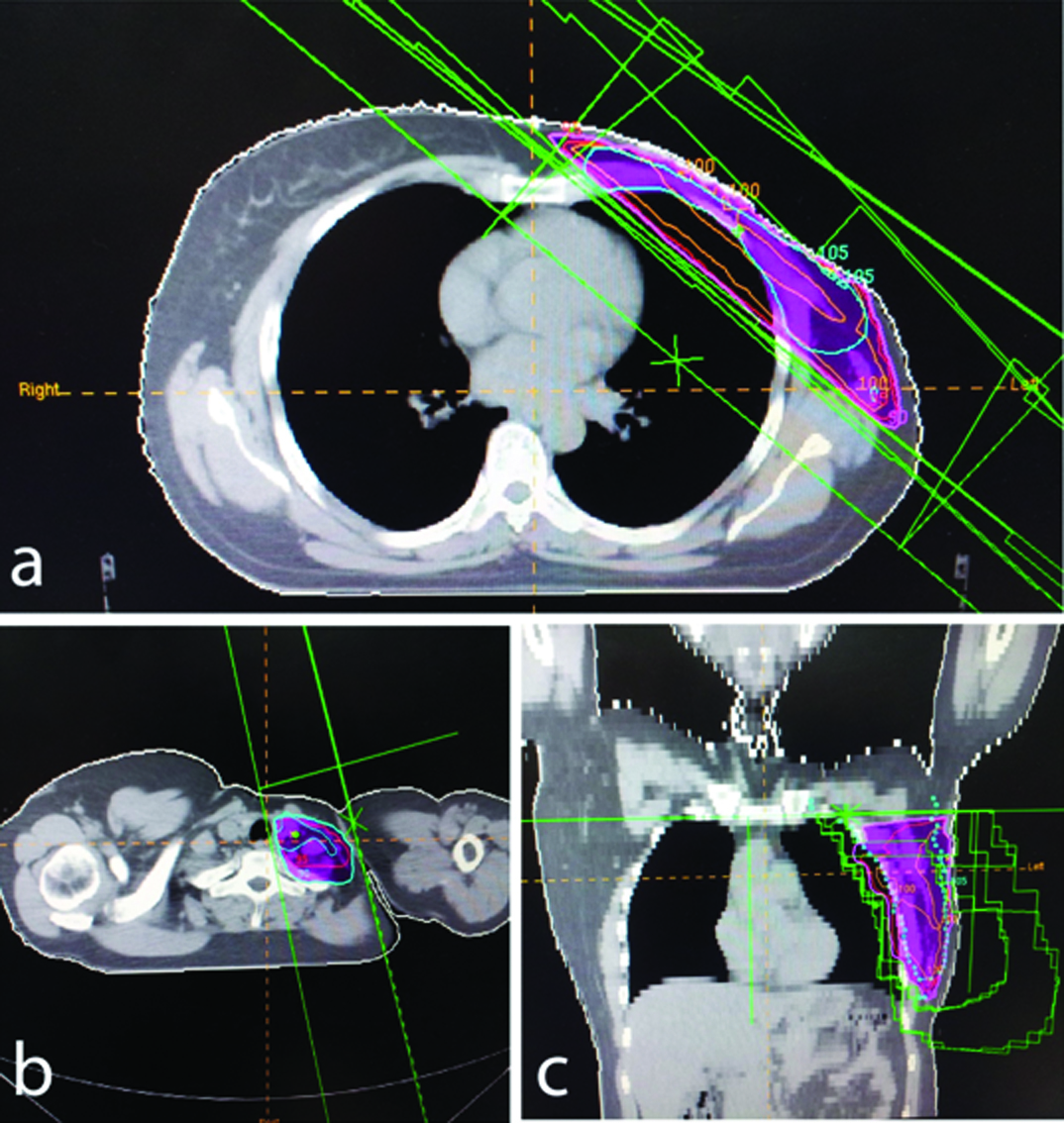
Image of CT planning and evaluation of a patient. a) Axial image of isodose evaluation of chest wall coverage by a 3-D conformal plan using wedges and ‘field-in field’ technique for reducing hot spots. b) single anterior beam to cover the supraclavicular fossa with angulation to avoid spinal cord and oesophagus. c) coronal section of isodose coverage.
All the patients had to undergo a baseline clinical examination before RT, and on a weekly basis while on treatment, followed thereafter by monthly evaluation for at least three months. During clinical visits, patients were assessed for development and severity of any acute toxicity including skin, haematological and pulmonary toxicities. All toxicity was graded according to RTOG Acute Radiation Morbidity Scoring Criteria. All data recording and analysis was done on Microsoft Excel 2007 and Statistical Package for Social Sciences (SPSS; version 20.0, IL, Chicago).
Results
A total of 56 patients meeting inclusion and exclusion criteria were enrolled in the study. All the patients were treated using HFRT. The median age of the patients was 49 years (range: 28-69 years). Almost 60% of the patients were under 50 years of age. The demographic details of the patients are shown in [Table/Fig-2].
Clinical profile of the patients included into the study.
| Patient related variable | Number (%) |
|---|
| Histology | |
| Infiltrating Ductal carcinoma | 53 (94.6) |
| Others | 3 (5.4) |
| Grade of tumour | |
| I | 8 (14.3) |
| II | 28 (50) |
| III | 20 (35.7) |
| Pathological T Stage | |
| Tx | 2 (3.6) |
| T1 - T2 | 41 (73.2) |
| T3 – T4 | 12 (21.4) |
| pT0 | 1 (1.8) |
| Pathological N stage | |
| N0 | 12 (21.4) |
| N1 | 30 (53.6) |
| N2-N3 | 14 (25) |
| Hormone receptor positivity | |
| Negative | 25 (44.6) |
| Positive | 31 (55.4) |
| Her2/neu status | |
| Negative | 38 (67.8) |
| Positive | 13 (23.2) |
| Not known | 5 (9) |
| Chemotherapy prior to RT | |
| Neoadjuvant | 5 (8.9) |
| Adjuvant | 44 (78.6) |
| No chemotherapy | 7 (12.5) |
| RT to the regional nodes | |
| Delivered | 39 (69.6) |
| Not delivered | 17 (30.4) |
Dosimetric evaluation
The average CTV was 371.3 cc (range: 231.7-610 cc) for patients receiving only chest wall RT, and 621.6 cc (range: 495 to 821 cc) for patients receiving supraclavicular and chest wall RT. The mean V90 and V95 to the PTV for the entire study population were 95% (95% CI: ± 3.3%) and 93% (95% CI: ± 4%) respectively. Similarly, the mean D90 was 40.8Gy (95% CI: ± 0.75 Gy). The average volume of PTV receiving more than 105% of the prescribed dose was 55 cc (range: 7.5-121 cc) for patients undergoing chest wall RT only and 94.9 cc (range: 53-152 cc) for patients receiving chest wall and supraclavicular RT. The maximum dose received was on an average 47.7 Gy (range: 46.2-48.5 Gy) for all the patients. The maximum dose was similar between patients undergoing chest wall (47.8 Gy) and chest wall with supraclavicular RT (47.7 Gy). On spatial evaluation, the target miss expectedly was at the medial and lateral edges of the PTV for all the patients.
The Mean Lung Dose of the entire study population was 10.2 Gy (± 3.5 Gy) and the mean V20 was 20.9% (± 6%). The mean heart doses among patients with left and right sided breast cancer were 4.3 Gy (± 2.2 Gy) and 1.0 Gy (± 0.2 Gy). On applying Independent-Samples t-test, this difference was statistically significant (p=0.001). Also, the mean value of V25 heart was 6.6% (± 4.8%) and 0% respectively for left and right sided breast cancers (p=0.001).
Acute toxicities of treatment
Prior to RT, 27 patients had grade I or higher anaemia. All of these patients had received prior chemotherapy. Over the course of RT, there was a partial recovery in anaemia. At the end of RT, 18 patients had grade I anaemia and one had grade II anaemia. No patient had worsening of anaemia while on RT. Also, no patient required blood transfusion for correction of anaemia. At one month after completion of RT, 15 patients had persisting anaemia, with two patients having grade II anaemia. Thrombocytopenia and neutropenia were not higher than grade II, and did not require any intervention or treatment interruption in any patient. Notably, patients who did not receive chemotherapy had no haematological toxicity. Haematological toxicity of the group is summarized in [Table/Fig-3].
Haematological toxicity following HFRT.
| Acute haematological toxicity | At completion | One month after RT | Three months after RT |
|---|
| Anaemia |
| Grade 0 | 37 | 39 | 42 |
| Grade I | 18 | 15 | 13 |
| ≥ Grade II | 1 | 2 | 1 |
| Leucocytopenia |
| Grade 0 | 37 | 39 | 42 |
| Grade I | 18 | 15 | 13 |
| ≥ Grade II | 1 | 2 | 1 |
| Thrombocytopenia |
| ≤ Grade I | 54 | 56 | 56 |
| ≥ Grade II | 2 | 0 | 0 |
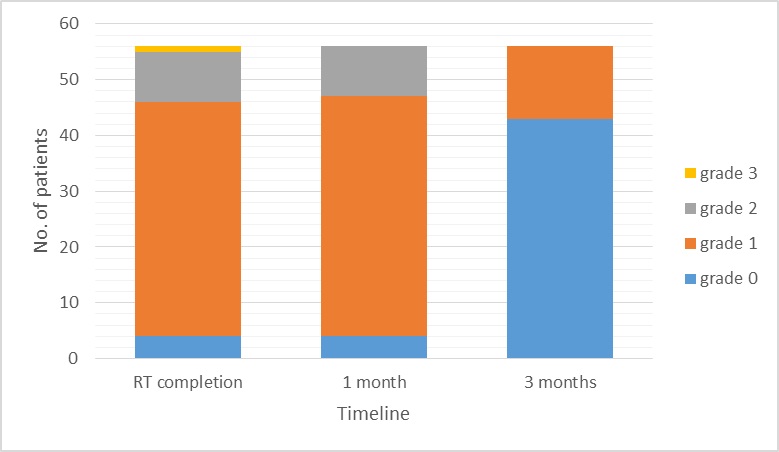
All patients were observed for skin toxicity as per the acute Radiation Therapy and Oncology Group (RTOG) morbidity scoring criteria. Majority of the patients had grade I (75%) and II (16%) toxicity. There was only one patient (1.8%) with grade III skin toxicity at the conclusion of RT. Four patients (7.1%) had developed no evidence of any dermatitis by the end of treatment. By one month after completion of RT, four (7.1%), 43 (76.8%) and nine (16.2%) patients had Grades 0, I and II acute radiation dermatitis, respectively. By the end of three months after completion of RT, skin reactions had subsided in 43 (76.8%) patients, while the remaining 13 patients (23.2%) had persisting grade I skin toxicity. By paired t-test, this recovery in dermatological toxicity was not statistically significant (p=0.62) [Table/Fig-4].
Recovery from acute skin toxicity after RT (p=0.62).
At the end of treatment, only three patients had developed mild (Grade I) dysphagia. All the three patients who had dysphagia at the end of radiotherapy belonged to the subset receiving chest wall and supraclavicular area. Only one patient had dysphagia persisting after one month of completion of RT, and no patient had swallowing problems after three months. None of our patients developed any symptomatic evidence of radiation pneumonitis at three months after completion of treatment.
Discussion
Although the equivalence of HFRT to conventional RT in terms of locoregional recurrence, survival and toxicity has been proven in four landmark trials [3,5–7], it is only slowly gaining popularity across the world. At our center, HFRT was adopted first in late 2014, and has been increasingly implemented since then. Presently, nearly all patients requiring adjuvant RT to the chest wall at our center undergo HFRT. This study was conducted to report on the early toxicity spectrum of HFRT.
In our study, PTV coverage was suboptimal, with volume receiving 95% of prescribed dose averaging 93%. None of the patients had a 100% V95. This underdosing is almost always a result of geometric miss of the PTV chest wall at the medial or lateral ends, as attempting to cover the entire PTV leads to beam passing through the contralateral breast, or excessive dose to lung and heart. For instance, in a report by Tanaka et al., the mean V90 and V95 seen with free breathing technique were 96.2% and 91%, respectively [8].
With hypofractionation, though there can be a reduction in mean lung dose as a consequence of lower total prescription dose, the V20 value is unlikely to be affected when compared to conventional fraction, because the identical planning technique (irrespective of fractionation schedule) ensures that volume coverage remains the same. Lung is believed to be very sensitive to both dose per fraction and total dose. The QUANTEC recommendations suggest not exceeding V20 by more than 35% and Mean Lung Dose (MLD) lower than 20–23 Gy to attain a <20% risk of pneumonitis [9]. Additional factors might also affect the risk of developing radiation pneumonitis. A recent report from Taiwan suggested that utilizing multiple variables including V20 to the ipsilateral lung, absolute volume of lung receiving greater than 20 Gy, age and low Body Mass Index (BMI) could better estimate the risk of pneumonitis [10]. Considering that the volume of lung receiving greater than 20 Gy is going to be irreversibly affected, the higher dose per fraction in a hypofractionation schedule is less relevant and the clinical outcomes in terms of pulmonary toxicity are likely to be the same between the two fractionation schedules. Our patients’ mean lung dose was within the recommended dose thresholds to the lung. Also, in the short follow-up duration, no patient developed any symptoms suggestive of radiation induced pulmonary toxicity.
Increase in the rates of cardiac morbidity and mortality following post mastectomy RT is an area of significant concern. It has been established that adjuvant RT to left sided breast cancers has a small but statistically significant increase in the risk of both cerebrovascular and cardiac deaths [11–13]. Moreover, there is potentially no threshold dose below which risk of cardiotoxicity is non-existent [14]. However, hypfractionation does not appear to pose a higher risk of cardiac complications when compared to conventional RT. For instance, in the report by Chan et al., with a median follow up of more than 14 years, the excess death due to cardiac causes was similar between conventional and HFRT (4.8 and 4.2%, respectively) [13]. On the other hand, there is a potential increase in risk of late cardiotoxicity with more extreme hypofractionation. A report by Tjessem et al., suggested that severe hypofractionation (43 Gy in 10 daily fractions) could increase the risk of cardiac injury [15]. Thus, caution must be exercised in adopting a more aggressively hypofractionated regimen especially if a considerable amount of heart is within the radiation fields.
Haematological toxicity secondary to RT was generally low, and is at least partially the result of prior chemotherapy received by the patient. Patients who did not receive prior chemotherapy had no neutropenia or thrombocytopenia during adjuvant RT. Contrary to expectation, there was a slight decrease in the incidence of anaemia during the course of RT. This is likely due to the gradual recovery from the accumulated haematological toxicity secondary to prior adjuvant chemotherapy.
Acute skin toxicity experienced by our patients was generally mild, with only one patient experiencing moist desquamation at the completion of treatment. Expectedly, the reactions peaked at the completion of treatment, and resolved over subsequent three months in more than 75% of the patients. In a similar report by Nandi et al., none of the patients developed grade IV acute reactions [16]. However, residual hyperpigmentation was reported beyond even six months. Another study comparing conventional and hypofractionated RT reported that conventional fractionation was associated with significantly higher rates of grade ≥2 dermatitis [17]. Acute skin toxicity with HFRT appears to be moderate and is usually self-limited, provided care is taken to avoid significant dose inhomogeneity. Data from retrospective studies suggest that large dose inhomogeneity (V > 107%) predisposes to more severe acute skin reactions [18,19]. Irrespective of the fractionation schedule used, Intensity Modulated RT has a superior capability of reducing acute skin toxicity, especially in patients following breast conservation surgery. In two randomized trials comparing IMRT to two-dimensional planning, improved dose profile after a more stringent evaluation for dose homogeneity resulted in decreased acute toxicity [20,21].
All three of the patients who developed mild dysphagia by the time of conclusion of RT had received supraclavicular RT. Dysphagia subsided completely in all patients by three months. Odynophagia is secondary to exposure of hypopharynx and cervical oesophagus to RT. The incidence of dysphagia could be reduced to some extent by appropriate angulation of the supraclavicular field.
Limitation
The scope of this study is significantly compromised by the small numbers of patients and short follow-up. However, dosimetric data suggest that accepted dose thresholds to the normal tissues, especially lung and heart, can be achieved in most patients. Also, none of our patients developed any serious acute toxicity during treatment that required medical intervention or treatment interruption. In view of the obvious benefits of shorter time and costs and strong evidence of clinical equivalence to conventional fractionation, adjuvant HFRT should be strongly considered as an option for patients requiring post mastectomy RT.
Conclusion
Adjuvant HFRT to the chest wall (with regional nodal RT when required) was found to be well tolerated, with mild to moderate acute adverse effects that did not warrant any therapeutic intervention or treatment interruption. The most common acute toxicity experienced was dermatitis, which resolved in most patients by three months. Myelosuppression was limited to patients who had received prior chemotherapy, and was generally mild.