An Increase Incidence in Uric Acid Nephrolithiasis: Changing Patterns
Asha Kumari1, Sumit Dokwal2, Pawan Mittal3, Rajender Kumar4, Richa Goel5, Piyush Bansal6, Himanshu Devender Kumar7, Jaikrit Bhutani8
1 Demonstrator, Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
2 Assistant Professor, Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
3 Medical Officer, ESIPinjore, Haryana, India.
4 Junior Resident, Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
5 Junior Resident, Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
6 Assistant Professor, Department of Biochemistry, BPS GMCKhanpur, Sonepat, Haryana, India.
7 Junior Resident, Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
8 House Surgeon, Department of Pulmonary and Critical Care Medicine, PGIMS, Rohtak, Haryana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sumit Dokwal, Assistant Professor, Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
E-mail: drsumitdokwal80@gmail.com
Introduction
Nephrolithiasis is a complex disease affecting all age groups globally. As the causative factors for nephrolithiasis rises significantly, its incidence, prevalence and recurrence continues to baffle clinicians and patients.
Aim
To study the prevalence of different types of renal stones extracted by Percutaneous Nephrolithotomy (PCNL) and open surgical procedures.
Materials and Methods
Renal stones from 50 patients were retrieved by Percutaneous Nephrolithotomy (PCNL), Ureterorenoscopy (URS) and open surgical techniques for qualitative tests for detection of calcium, oxalate, uric acid, phosphate, ammonium ion, carbonate, cystine and xanthine.
Results
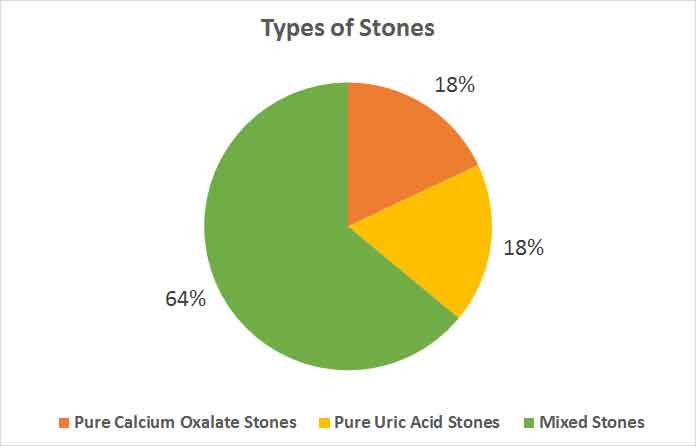
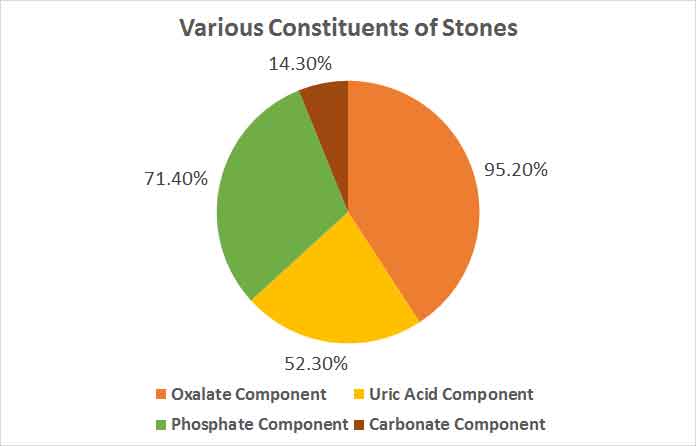
Three patients had stone removed by open surgery and rest had undergone PCNL. Nine of the stones were pure of calcium oxalate, 9 were of pure uric acid and 32 were mixed stones. Forty one stones had calcium. Among the mixed stones, oxalate was present in 25 samples (39 of total), uric acid was seen in 17 (25 of total stones), phosphate was present in 23 (23 of total) and carbonate was present in 4 stones (4 of total). Only 1 patient had triple phosphate stone. 12 were of staghorn appearance of which 6 were of struvite type, 6 were pure uric acid and remaining were mixed oxalate-phosphate stones.
Conclusion
Our study, though in a small number of hospital based patients, found much higher prevalence of uric acid stones and mixed stones than reported by previous hospital based studies in north India (oxalate stones~90%, uric acid~1% and mixed stones~3%). Biochemical analysis of renal stones is warranted in all cases.
Renal stones, Stone composition, PCNL, Oxalate, Paradigm shift in renal stones
Introduction
A heterogeneous distribution of renal stones seen throughout the world marks certain areas as stone belt. This belt encompasses Sudan, the Arab Republic of Egypt, Saudi Arabia, the United Arab Emirates, the Islamic Republic of Iran, Pakistan, India, Myanmar, Thailand and Indonesia to Philippines [1].
In India, the incidence of renal calculi is comparatively low in the southern part of country [2] and high prevalence areas include Maharashtra (7.6%) and Rajasthan [3–5]. Calcium oxalate has been found as the predominant constituent in nephrolithiasis in India [2,6].
Renal calculus disease (RCD) has been found to have a recurrence of 26-53% after a decade of follow-up [7–9]. Studies indicate that primary stone formation is usually associated with one or more metabolic derangements [10,11]. However, due to lack of algorithm based evaluation by clinicians, a thorough metabolic workup is overlooked in these patients [12]. Additionally, metabolic evaluation followed by selective therapy has shown to decrease the incidence of formation of new calculi [13,14]. The frequency and type is variable depending upon different climates [15,16] and in racial groups [17].
A study done in Saudi Arabia found a strong correlation between urinary stone colic and both temperature and atmospheric pressure. The results showed a steady increase in urinary stone colic in the hot season with a maximum rate in the months of June, July and August [15]. High temperature promoted stone formation in men working in steel industry in another study [16]. Soucie et al., in a large cross-sectional survey of 25,286 U.S. adults in the United States found that the prevalence of kidney stones was highest among White people and lowest in Black people. Hispanic and Asian people had an intermediate prevalence [18].
Knowledge of stone composition of a patient can help in unveiling aetiology, suggesting most suitable treatment modality and predicting chances of recurrence. It can thus improve the patient management.
Strauss et al., reported in Caucasians that 51% of the subjects had idiopathic hypercalciuria or hyperuricosuria, while 19.8% had a systemic disorder producing stones [19]. Pak [10] reported absorptive hypercalciuria in 55.9%, renal hypercalciuria in 11.8%, primary hyperparathyroidism in 2.9%, hyperuricosuric calcium oxalate nephrolithiasis in 8.8% and no metabolic abnormality in 20.6% of their patients.
Hosseini et al., reported from Iran that the most common abnormality observed was low 24 hour urine volume (58.24%), followed by hypercalciuria (17.18%) and hyperuricosuria (15.15%) [11]. These metabolic derangements favours recurrence. Outcome by treatment modalities like shock wave monotherapy is affected by stone number, composition and location also [20].
Materials and Methods
This cross-sectional study was conducted during a period of six months from Jan 2013 to June 2013. Renal stones were retrieved surgically by percutaneous nephrolithotomy (PCNL), ureterorenoscopy (URS) and open surgeries after informed written consent from 50 patients coming to outpatient Department of Urology, Postgraduate Institute of Medical Sciences, Rohtak, India. Presence of renal stone was indicated by a history of renal colic, decrease in urine volume or hematuria and confirmed by ultrasonography, X-ray KUB. Computerized tomography was used in some radiolucent renal stones. Immobilized patients, patients with bowel disorders and those on drugs like calcium, antacids and vitamins were excluded. Past history of any metabolic or endocrinal disorder was elicited. Dietary history related to non-vegetarian and food rich in oxalates and calcium, hard water intake, amount of fluid intake, family history along with anthropometric measurements were recorded.
After the surgery, stone was collected in a sterile container without any preservative and sent directly to Department of Biochemistry for qualitative analysis. Stone was washed with distilled water three times to remove any blood stains. Complete drying was done in hot air oven at 60°C for five hours. Each stone was subjected to gross examination for size, colour, surface, concentric rings and consistency. Stone was grounded to fine powder by mortar and pestle and then qualitative tests for calcium, oxalate, uric acid, phosphate, ammonium ion, carbonate, cystine and xanthine were done. Murexide test was done for Uric acid and ammonium urate. Xanthine does not give murexide test but gives red or orange colour instead. Hexagonal cystine crystals were searched microscopically [21]. Oxalate, carbonate and phosphates were tested by adding dilute HCl on powder of stone and changing pH by ammonia and acetic acid sequentially. Phosphates, cystine and oxalates dissolve in dilute HCl, which after alkanization with ammonia leads to precipitation of oxalates and phosphates. Oxalates and phosphates are differentiated by acetic acid which dissolves phosphates. Ammonium molybdate test was done for phosphates while Nessler’s reagent was used for Ammonia detection. Calcium was detected with ammonium oxalate and magnesium with titan yellow [21].
Results
Mean age group of patients was 33.2±9.3 years. The mean duration from diagnosis of renal stone to evaluation was 12.3 ± 12.2 months (range 1-51 month). Seventeen patients gave family history of renal stone in first-degree relatives. Three patients had stone removed by open surgery and rest had undergone PCNL. Out of stones from 50 patients analysed 9 were pure calcium oxalate (18%), 9 were of pure uric acid (18%) and 32 were mixed stones (64%) [Table/Fig-1]. The composition of all stones and their relative occurrences among each other are shown in [Table/Fig-2]. Only 1 patient had triple phosphate stone. Twelve patients had staghorn stone in appearance of which 6 were of struvite type, 6 had pure uric acid and remaining were mixed oxalate-phosphate stones.
Various constituents of stones.
Discussion
The study was conducted to find the prevalence of uric acid stones among renal stones extracted by PCNL and open surgical procedures. We found a higher incidence of uric acid pure stones (18%) and mixed stones containing uric acid (52.3%) in comparison to previous studies. Mixed stones were the most prevalent ones with calcium oxalate as a component in more than 90% of them [6,22,23] [Table/Fig-3].
Calcium oxalate stone percentage in various studies.
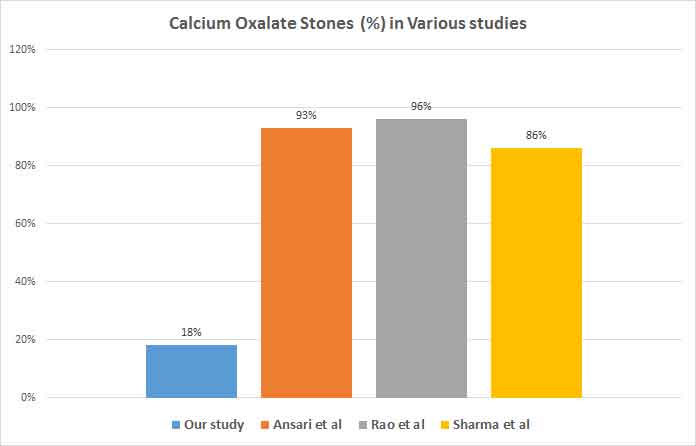
A study conducted by Ansari et al., in north India among 1050 renal stones reported calcium oxalate (n = 977) in 93.04% patients, uric acid stones (n=10; 0.95%) and rest had mixed pattern stones (n = 29; 2.76%) [6].
Another study done by Rao et al., in Delhi found that 96% of stones out of 51 stones analysed were calcium oxalate stones [22] Similarly, Ahlawat et al., and Sharma et al., observed that 97% and 86.1% of stones were composed of calcium oxalate [23,24].
Quantitative analyses of urinary stones by FT-Raman spectroscopy show that calcium oxalate monohydrate (40%), apatite (30%), magnesium ammonium phosphate (23%) and uric acid (7%) are present in all the urinary stone samples. Annamalai University, Tamil Nadu, India [25].
Studies in western countries have reported different pattern of stone variety prevalence. Maalouf et al., found that UA nephrolithiasis comprises 8-10 % of all kidney stones in the United States, 16% of stones in Okinawa, Japan, and 25% of stones in certain regions in Germany [26]. Daudon et al., reported an incidence of 65.98% calcium oxalate stones in France while Herring et al., showed the incidence of COM and COD to be 31.4% and 40.9% [27,28].
Pathologies behind uric acid stones can be congenital, acquired or idiopathic. Recently a gene has been linked to uric acid stone formation but further studies are required for proving genetic linkage [29] Low urinary pH, hyperuricosuria and low urinary volume are the key aetiologic factors held responsible for uric acid nephrolithiasis. High temperature regions like north India provide favourable conditions by decreasing urine volume and pH. Decrease in urine volume (<2 litres/day) increases urinary saturation of calcium oxalate, calcium phosphate and uric acid facilitating stone formation [26].
Studies in the past have indicated that increase in Net Acid Excretion (NAE) and decrease in renal ammonium (NH4+) leads to increased urinary. This decreased renal ammonium clearance has been shown to exist in a steady state 24 hour urine sample. Additionally, an oral ammonium chloride (NH4CL) challenge amplifies these ammoniogenic defects. However, unfortunately the detailed underlying mechanisms of increased acid generation, and the source and nature of responsible organic ions, remains less described. One plausible mechanism is the production of organic acid by intestinal and aerobic metabolism. This may occur in obese, diabetic and uric acid stone formers due to the differences in gut microflora [30]. Type II Diabetics and obese whose numbers is on exponential increase in India are also more prone for UA nephrolithiasis.
Indians are different from western population in respect of dietary habits, climate and genetic makeup. Nutritionally, poor diet that is low in animal protein, calcium and phosphate, but high in cereal and is acidogenic [31]. This leads to the formation of urine with a relatively high content of ammonium and urate ions and, consequently, to the formation of ammonium acid urate crystals and stones. Low urine volumes resulting in some areas from poor drinking water, which causes chronic diarrhea, and in others from the hot climate and fluid losses through the skin decreases urine volume [31].
In the literature it has been described that children in India who consumed a predominant wheat diet (staple food in North India) have increased uric acid precipitation and saturation thus predisposing them to stone formation [32].
Limitation
This was a cross-sectional observational study and no follow-up for recurrent stones was done. Although the study was carried out for 6 months, the sample of this study was limited. Thus, higher prevalence of UA stones, needs to be researched further. Also, this study assessed biochemical composition of stones retrieved by open surgery or PCNL, while smaller stones removed using other available methods were not assessed.
Conclusion
Uric acid nephrolithiasis is more common in north India. Patients of renal calculi should be investigated for serum uric acid and urine pH on routine basis. Uric acid stones also recur more frequently which can be prevented by balancing urine pH, modifying diet and increasing fluid intake.
[1]. Hussain M, Lal M, Ahmed S, Zafar N, Naqvi SA, Abid-ul-Hassan Management of urinary calculi associated with renal failureJ Pak Med Assoc 1995 45(8):205-08. [Google Scholar]
[2]. Pendse AK, Singh PP, The aetiology of urolithiasis in udaipur (Western Part of India)Urol Res 1986 14:59-62. [Google Scholar]
[3]. Bakane BC, Nagtilak SB, Bhaidas P, Urolithiasis in satpura region: a tribal ExperienceInternational Med J 1996 3:215-17. [Google Scholar]
[4]. Pendse AK, Urolithiasis in Udaipur and Jodhpur. A comparative study on prevalence and urinary profileBull III, Ann Conf Urol Soc India 1985 :12 [Google Scholar]
[5]. Coe FL, Parks JH, Asplin JR, The pathogenesis and treatment of kidney stonesN Engl J Med 1992 327:1141-52. [Google Scholar]
[6]. Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M, Spectrum of stone composition: structural analysis of 1050 upper urinary tract calculi from northern IndiaInt J Urol 215 12(1):12-16. [Google Scholar]
[7]. Trinchieri A, Ostini F, Nespoli R, Rovera F, Montanari E, Zanetti G, A prospective study of recurrence rate and risk factors for recurrence after a first renal stoneJ Urol 1999 162:27-30. [Google Scholar]
[8]. Ljunghall S, Danielson BG, A prospective study of renal stone recurrencesBr J Urol 1984 56:122-24. [Google Scholar]
[9]. Ahlstrand C, Tiselius HG, Recurrences during a 10-year follow-up after first renal stone episodeUrol Res 1990 18:397-99. [Google Scholar]
[10]. Pak CY, Should patients with single renal stone occurrence undergo diagnostic evaluation?J Urol 1982 127:855-58. [Google Scholar]
[11]. Hosseini MM, Eshraghian A, Dehghanian I, Irani D, Amini M, Metabolic abnormalities in patients with nephrolithiasis: Comparison of first-episode with recurrent cases in Southern IranInt Urol Nephrol 2010 42:127-31. [Google Scholar]
[12]. Hughes P, The CARI guidelines. Kidney stones: Metabolic evaluationNephrology (Carlton) 217 12(Suppl 1):S31-33. [Google Scholar]
[13]. Pearle MS, Roehrborn CG, Pak CY, Meta-analysis of randomized trials for medical prevention of calcium oxalate nephrolithiasisJ Endourol 1999 13:679-85. [Google Scholar]
[14]. Pak CY, Pharmacotherapy of kidney stonesExpert Opin Pharmacother 218 9:1509-18. [Google Scholar]
[15]. Al-Hadramy MS, Seasonal variations of urinary stone colic in ArabiaJ Pak Med Assoc 1997 47:281-84. [Google Scholar]
[16]. Atan L, Andreoni C, Ortiz V, Silva EK, Pitta R, Atan F, High kidney stone risk in men working in steel industry at hot temperaturesUrology 215 65(5):858-61. [Google Scholar]
[17]. Hughes P, The CARI guidelines. Kidney stones epidemiologyNephrology (Carlton) 217 12(Suppl1):S26-30. [Google Scholar]
[18]. Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H, Demographic and geographic variability of kidney stones in the United StatesKidney Int 1994 46:893-99. [Google Scholar]
[19]. Strauss AL, Coe FL, Parks JH, Formation of a single calcium stone of renal origin. Clinical and laboratorycharacteristics of patientsArch Intern Med 1982 142:5047 [Google Scholar]
[20]. Skolarikos A, Mitsogiannis H, Deliveliotis C, Indications, prediction of success and methods to improve outcome of shock wave lithotripsy of renal and upper ureteral calculiArch Ital Urol Androl 2010 82(1):56-63. [Google Scholar]
[21]. Varley H, Practical Clinical Biochemistry 1988 Fourth EditionNew DelhiCBS Publishers and Distributors [Google Scholar]
[22]. Rao MVR, Agawan JS, Tania OP, Studies in urolithiasis II: X-ray diffraction analysis of renal calculi from Delhi regionIndian J Med Res 1976 64:102 [Google Scholar]
[23]. Ahlawat R, Goel MC, Elhence A, Upper urinary tract analysis using X-ray diffraction: results from tertiary referral center in North IndiaNatl Med J India 1996 9:10-12. [Google Scholar]
[24]. Sharma RN, Shah I, Gupta S, Thermogravimetric analysis of urinary stonesBr J Urol 1989 64:10-13. [Google Scholar]
[25]. Selvaraju R, Raja A, Thiruppathi G, FT-Raman spectral analysis of human urinary stonesSpectrochim Acta A Mol Biomol Spectrosc 2012 99:205-10. [Google Scholar]
[26]. Maalouf NM, Cameron MA, Moe OW, Sakhaee K, Novel insights into the pathogenesis of uric acid nephrolithiasisCurr Opin Nephrol Hypertens 214 13:181-89. [Google Scholar]
[27]. Daudon M, [Epidemiology of nephrolithiasis in France]Ann Urol (Paris) 215 39(6):209-31. [Google Scholar]
[28]. Herring LC, Observation of 10,10 urinary calculiJ Urol 1962 88:545-62. [Google Scholar]
[29]. Gianfrancesco F, Esposito T, Ombra MN, Identification of a novel gene and a common variant associated with uric acid nephrolithiasis in a Sardinian genetic isolateAm J Hum Genet 213 72:1479-91. [Google Scholar]
[30]. Sakhaee K, Epidemiology and clinical pathophysiology of uric acid kidney stonesJ Nephrol 2014 27(3):241-45. [Google Scholar]
[31]. Robertson WG, Renal stones in the tropicsSemin Nephrol 213 23:77-87. [Google Scholar]
[32]. Teotia M, Krishna S, Teotia SP, Kidney and vesical stones in children Chapter 106. In: Teotia SP, Teotia M, editorsNutritional and Metabolic Bone and Stone Disease An Asian Perspective 2008 New DelhiCBS Publishers and Distributors:795-807. [Google Scholar]