Rare Case of Unilateral Hypoplasia of Lung with Associated Ventricular Mass in an Adult
Lavina Vishnu Mirchandani1, Azad Alam2, Aparna Iyer3, Jayalakshmi Thelapurath Kutty4
1 Associate Professor, Department of Pulmonary Medicine, Dr. D.Y. Patil University, College of Medicine, Nerul, Navi Mumbai, Maharashtra, India.
2 Resident, Department of Pulmonary Medicine, Dr. D.Y. Patil University, College of Medicine, Nerul, Navi Mumbai, Maharashtra, India.
3 Assistant Professor, Department of Pulmonary Medicine, Dr. D.Y. Patil University, College of Medicine, Nerul, Navi Mumbai, Maharashtra, India.
4 Professor and Head, Department of Pulmonary Medicine, Dr. D.Y. Patil University, College of Medicine, Nerul, Navi Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Lavina Vishnu Mirchandani, Shyam Kutir CHS, Flat # 302, 13TH Road, TPS III, Plot 629, Khar (west), Mumbai- 400052, Maharashtra, India.
E-mail: drlavinamir@gmail.com
Hypoplasia of the lung is a rare congenital condition which can be: a) primary i.e. no apparent cause is found; or b) secondary i.e. associated with other congenital anomalies that are implicated in its pathogenesis. These anomalies may involve the diaphragm, cardiovascular, central nervous, urogenital and musculoskeletal system. Patients usually present in neonatal, infancy or childhood period and very rarely in adulthood. Our patient was an adult having a unilateral hypoplastic lung associated with a ventricular mass and to our knowledge this rare combination has never been reported in the English literature; though there are reports of prenatal or newborns with hypoplastic lung and rhabdomyoma of ventricle who did not survive.
Cardiac tumour, Congenital lesions, Developmental lung disorder
Case Report
A 69-year-old male, non-smoker, farmer presented to us for the first time with low grade fever and cough with mucopurulent expectoration, since 10 days. Patient had dyspnoea since 5 years, worsened since 10 days, not associated with orthopnoea or paroxysmal nocturnal dyspnoea. The patient had past history of similar respiratory symptoms 45 and 40 years ago and according to him, was treated on both occasions with antituberculous drugs based on chest X-ray findings. No old reports, papers, or investigations were available with the patient and he had remained almost asymptomatic till about 5 years ago.
Systemic examination showed that he was febrile and had tachycardia. Respiratory examination revealed reduced movements of the left hemi thorax, tracheal and mediastinal shift to the left, rib crowding and reduced breath sounds in the left hemi thorax with bilateral rhonchi and crepitations. Apex beat was felt in the posterior axillary line on the left, but heart sounds were normal and no murmur was heard.
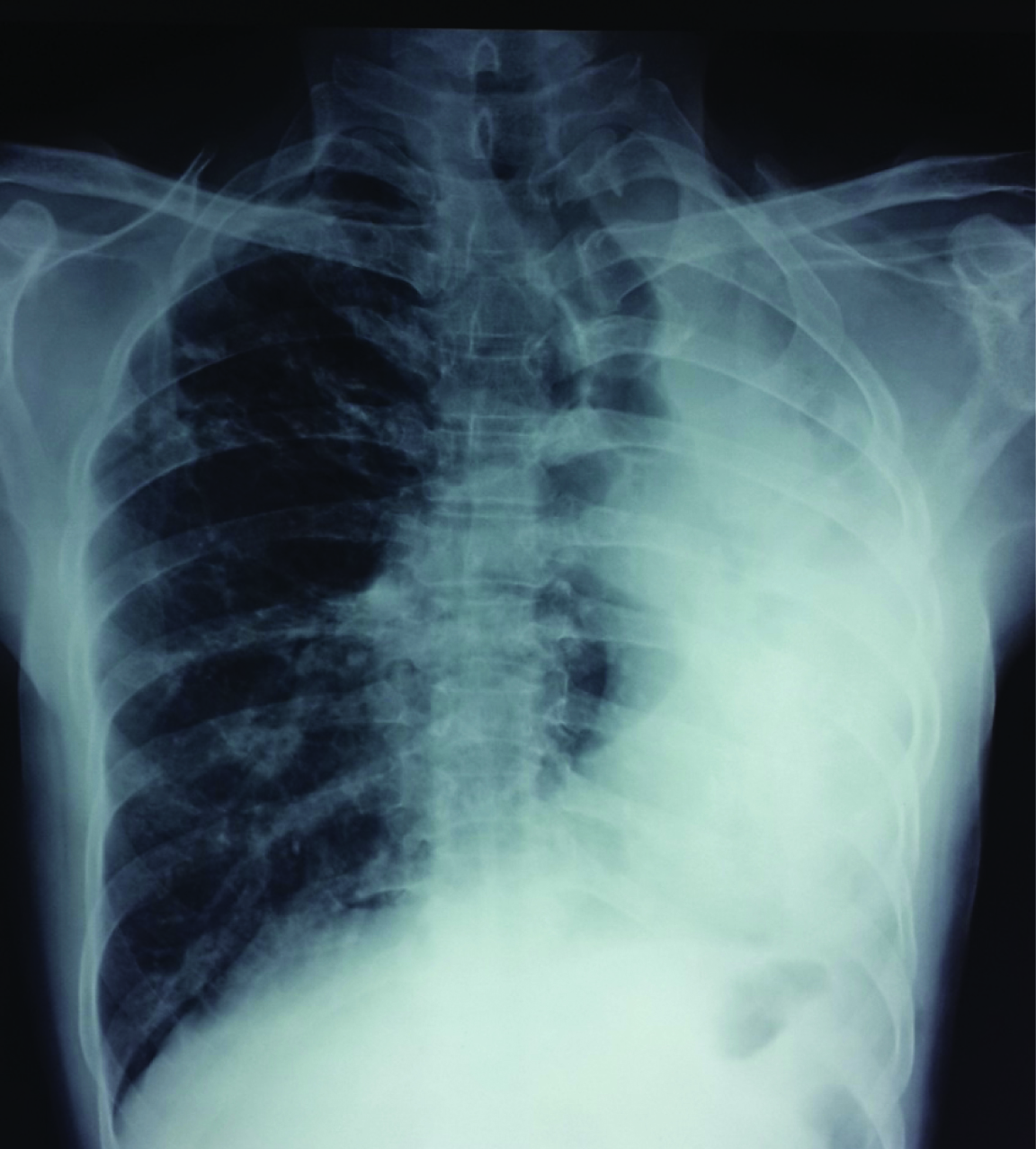
Chest radiograph showed tracheal and mediastinal shift to the left, with complete opacification and volume loss of left hemithorax, and compensatory emphysema and herniation of the right lung [Table/Fig-1]. Routine blood investigations were normal. Mycobacterium tuberculosis was not detected in sputum smear or by Genexpert. Sputum bacterial culture grew Streptococcus species.
Posteroanterior chest radiograph showing opacification of the left hemithorax with decrease in size and mediastinal shift to the left with an increase in the volume of the right lung. The heart outline is indistinct.
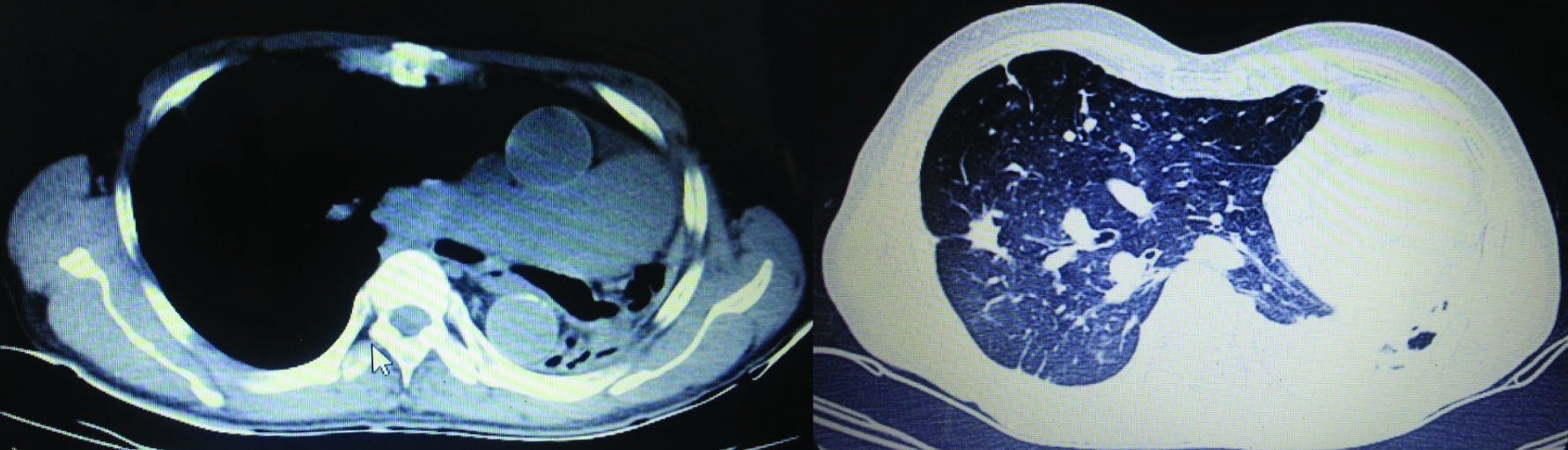
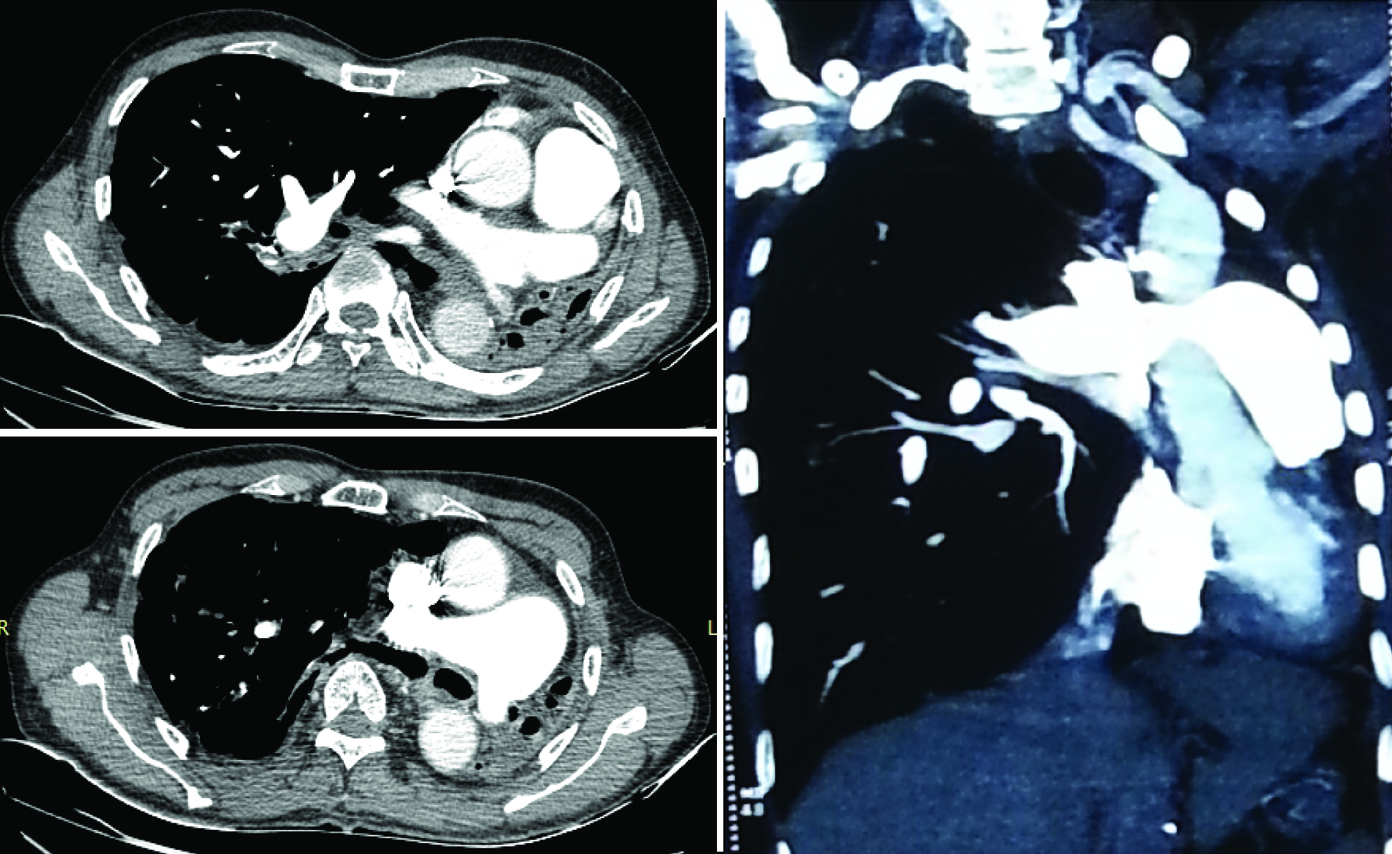
High Resolution Computed Tomography (HRCT) of chest revealed complete collapse of left upper and lower lobe with multiple pleural and parenchymal calcifications, volume loss signs in left hemithorax and herniation of right lung to the contralateral side and traction bronchiectactic changes in some areas of the right lung. A decrease in size and cut off of left main bronchus were noted [Table/Fig-2]. CT pulmonary angiography showed cut off of the left pulmonary artery but with no evidence of thrombus, thus confirming the diagnosis of left lung hypoplasia. There was narrowing of the left pulmonary artery in the collapsed lung, with two small branches arising from the pulmonary artery [Table/Fig-3].
Plain CT (mediastinal and lung window respectively) showing hypoplastic left lung with mediastinal displacement to the left. Decreased size and cut off of left main stem bronchus seen. Hyper inflated right lung seen herniated to the contralateral side.
CT Pulmonary angiography axial and coronal sections show abrupt cut off of the left pulmonary artery in the collapsed lung. No evidence of thrombosis seen. Mediastinal shift to left and reduced size of left hemithorax with herniation of right lung seen.
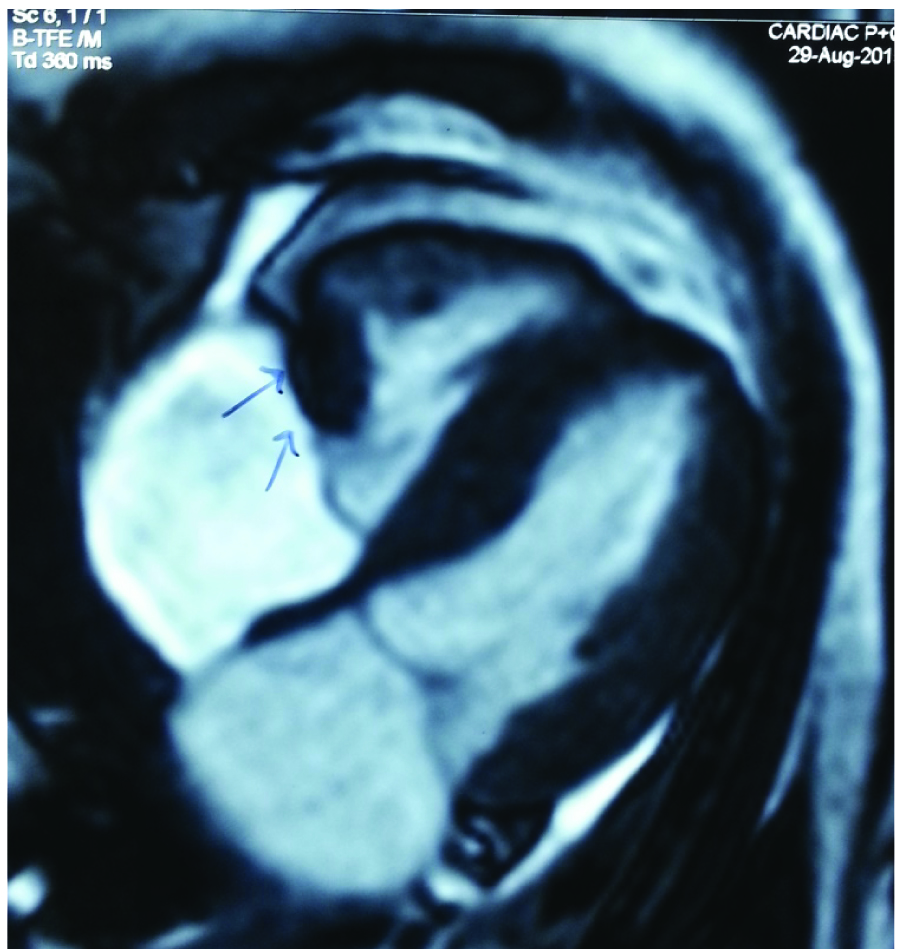
ECG showed sinus tachycardia, right axis deviation with partial right bundle branch block. Persistent tachycardia and an observation that there was a change in the heart rate on posture led us to investigate this patient for cardiac anomalies. Two Dimensional ECHO revealed normal chambers but vegetation was reported in the anterior tricuspid valve. Target Scan to rule out DVT was normal. Transoesophageal echocardiography and Cardiac MRI revealed a mass lesion (19 x14mm) arising from the free wall of right ventricle, abutting the tricuspid valve during systole [Table/Fig-4]. The signal intensity characteristics and physiological features favoured fibroma/pseudo tumour.
Cardiac MRI shows mass lesion arising from free wall of right ventricle.
Mean while patient was treated with antibiotics as per sputum culture sensitivity, mucolytic, chest physiotherapy, inhaled bronchodilators and inhaled steroids. He responded well to treatment and was discharged subsequently. Cardiologists opined that no active management of the cardiac lesion was required presently as patient had no cardiac symptoms, and asked patient to follow-up initially every month for evaluation. Preventive vaccination against influenza virus and pneumococcus was given and patient was asked to follow up regularly.
Discussion
Pulmonary hypoplasia is a rare congenital disorder of lung development, the prevalence of which is about 7 to 26% of all neonatal autopsies [1]. Unilateral pulmonary hypoplasia prevalence is 1-2/12,000 or 15,000 births [2], though this may be an underestimation. In almost 70% cases the left lung is affected [3]. Pulmonary hypoplasia is usually secondary to other congenital abnormalities like diaphragmatic hernia, vascular or thoracic cage anomalies, oligohydramnios, maternal treatment with ACE inhibitors, etc. Primary pulmonary hypoplasia presenting in an adult is extremely rare and this is probably the first case reporting its association with a cardiac tumour in an adult. Though no apparent cause is implicated in the pathogenesis of primary pulmonary hypoplasia, it can rarely be associated with cardiac and vascular anomalies such as unilateral absence of the pulmonary artery, cardiac tumours and other congenital heart diseases. Since these are early errors of development, whether they are cause, effect or an association is difficult to speculate.
In 1912 Schneider [4] classified lung maldevelopment into three groups, modified in 1955 by Boyden [5] as:
Type I (Agenesis): complete absence of parenchymal tissue bronchial and vascular supply.
Type II (Aplasia): absence of parenchymal tissue but a rudimentary bronchus present, no evidence of pulmonary vasculature.
Type III (Hypoplasia): presence of variable amounts of lung parenchyma with decreased number or size of airways, vessels, and alveoli.
Pulmonary hypoplasia was classified in 1960 by Monaldi [6] as:
Group 1: No bifurcation of the trachea.
Group 2: Only rudimentary bronchus.
Group 3: Incomplete development after division of the main bronchus.
Group 4: Incomplete development of sub segmental bronchi and a small segment of corresponding lobe.
In humans, lung development starts at 3 weeks of embryonic life and continues up to 8 years [7]. If the pathological process affecting the lung bud occurs during the embryonic stage i.e. upto around 5th week, it results in agenesis, but if it occurs in the later stages it results in aplasia or hypoplasia. By definition, primary hypoplasia is unassociated with other anomalies which can be implicated in its pathogenesis. Autosomal recessive chromosomal aberration, deficiency of vitamin A, intrauterine infections, environmental factors have been held responsible for it [8]. Patients usually present in childhood with respiratory distress or recurrent infection or haemoptysis. Rarely they present in adulthood, as in our case. Chest X-ray findings of complete opacification of hemithorax with volume loss prompt differential diagnoses of suspected foreign body aspiration, mucus plug occlusion, bronchial mass lesions and post infectious collapse. HRCT with CT pulmonary angiography confirm the diagnosis, as in our case.
Our elderly male patient had primary left lung hypoplasia of Monaldi group 3 associated with right ventricular mass, probably a fibroma. Primary cardiac tumours are extremely rare and have an estimated autopsy prevalence of 0.001-0.03% [9]. Cardiac myxoma (50%) and lipoma (10%) are the commonest benign tumours in adults. Association of pulmonary hypoplasia with a cardiac tumour in an adult, as seen in our case, has never been reported in English literature to the best of our knowledge; though there are reports of prenatal or newborns with hypoplastic lung and rhabdomyoma of ventricle who did not survive [10].
Adult patients with hypoplasia are treated conservatively with antibiotics for infections, symptomatic treatment with bronchodilators and management of complications like haemoptysis. Prophylactic pneumococcal and influenza vaccinations are recommended. Prognosis depends on associated congenital anomalies and the normalcy or otherwise of the other lung.
Conclusion
This is probably the first case reporting adult presentation of unilateral lung hypoplasia with a ventricular mass. The possibility of hypoplasia must be borne in mind in chest X-rays showing an opaque or partially opaque hemithorax with signs of volume loss. Cardiac evaluation should be routinely done in every patient with respiratory complaints.
[1]. Abrams ME, Ackerman VL, Engle WA, Primary unilateral pulmonary hypoplasia: neonate through early childhood — case report, radiographic diagnosis and review of the literatureJournal of Perinatology 2004 24:667-70. [Google Scholar]
[2]. Devi YG, Rani NU, Rao GS, Narayana M, Kumar BD, A rare clinical presentation of primary pulmonary hypoplasia with tuberculous pleural effusionIJSS Case Reports & Reviews 2014 1(3):16-19. [Google Scholar]
[3]. Dobremez E, Fayon M, Vergnes P, Right pulmonary agenesis associated with remaining bronchus stenosis, an equivalent of postpneumonectomy syndrome. Treatment by insertion of tissue expander in the thoracic cavityPediatr Surg Int 2005 21:121-22. [Google Scholar]
[4]. Schneider P, Schwatbe E, Part 2. Die Morphologie der Missbildungen Des Menschen Under Thiere 1912 vol. 3JenaGustav Fischar:817e822 [Google Scholar]
[5]. Boyden E, Developmental anomalies of lungAm J Surg 1955 89:79e88 [Google Scholar]
[6]. Monaldi V, Malformative bronchopulmonary diseases caused by anatomical defectsMinerva Med 1960 51:3474-78. [Google Scholar]
[7]. Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, Development and growth of the lungFishman’s Pulmonary Diseases and Disorders 2008 5(4):91-114. [Google Scholar]
[8]. Roy PP, Datta S, Sarkar A, Das A, Das S, Unilateral pulmonary agenesis presenting in adulthoodRespiratory Medicine Case Reports 2012 5:81e83 [Google Scholar]
[9]. Grebenc ML, Rosado de Christenson ML, Burke AP, Primary cardiac and pericardial neoplasms: radiologic-pathologic correlationRadiographics 2000 20(4):1073-103. [Google Scholar]
[10]. Kagan KO, Schmidt M, Kuhn U, Kimmig R, Ventricular outflow obstruction, valve aplasia, bradyarrhythmia, pulmonary hypoplasia and non-immune fetal hydrops because of a large rhabdomyoma in a case of unknown tuberous sclerosis: a prenatal diagnosed cardiac rhabdomyoma with multiple symptomsBJOG: an International Journal of Obstetrics and Gynaecology 2004 111:1478-80. [Google Scholar]