Chronic Myeloid Leukaemia (CML), a Myeloproliferative Neoplasm (MPN) characterized by Philadelphia chromosome accounts for 15% of adult Leukaemias. CML occurs in 3 phases namely chronic, accelerated and blast phase and untreated chronic phase, which eventually transforms into advanced phase in 3-5 years. Outlook of CML changed considerably with the introduction of tyrosine kinase inhibitors (TKI)-Imatinib initially followed by newer generation drugs like Dasatinib, Nilotinib, Bosutinib and Ponatinib [1]. However, Thrombo-haemorrhagic events remain an important cause of morbidity and mortality in patients of CML [2]. Review of the literature revealed that all the components of coagulation cascade are disturbed in CML to varying extents. As compared to other myeloproliferative disorders, derangement of haemostasis is less common in CML [3]. Both hyperleucostasis and drugs (Dasatinib) have been shown to affect the haemostatic pathway in CML [4,5]. However, the correlation between haemostatic abnormalities and phase of the CML as well as the effect of Imatinib on these abnormalities is unclear in the literature.
Present study aimed to study the various haemostatic parameters in patients with CML (on treatment with imatinib) and to draw correlation of these parameters with the phases of the disease as well as the response to the treatment.
Materials and Methods
The study was conducted on the CML patients attending the hematology clinic of a tertiary hospital in India. The duration of study was from October 2012 to April 2014 and was a cross-sectional study. A written informed consent was taken from all the patients prior to their inclusion in study. The authors declare that all procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study population included 30 patients with CML, 13 years of age and above of either sex (sample size was based on the burden of CML cases at our centre). Patients with any previously diagnosed haemostatic disorder, any family history of bleeding disorder, taking drugs known to affect haemostatic system, any liver disease, renal disease, any systemic infection likely to affect haemostasis, pregnant patients, those taking Dasatinib and patients not willing to give their consent were excluded from the study. Patients were subjected to detailed history and clinical examination and diagnosis of CML was made on clinical examination, peripheral blood smear, bone marrow examination and detection of bcr-abl gene product in peripheral blood and patients were then started on treatment. As per the protocol, patients who received imatinib as part of their treatment were included in the study and patients on other alternative treatments including Dasatinib were excluded. Complete blood counts, PT, APTT, D-dimers, fibrinogen, coagulation factor VIII levels and bcr-abl gene product- quantitative were assayed on venous blood sample of the patients. Complete blood counts were calculated using automated electronic cell counter. Coagulation parameters including PT, APTT, Fibrinogen, D-dimers and Factor VIII level were assayed by standardized methods. A 2.7ml blood was drawn in a trisodium citrate anticoagulant vial. The blood samples were centrifuged for 15min at 3000rpm and plasma was collected in appendorf tubes. Plasma was stored at -800C in refrigerator till use. Samples were analysed within 48hours for PT, APTT, Fibrinogen and Factor VIII levels by the standardized procedures. PT was measured by one stage assay by mixing platelet poor plasma with phospholipid containing thromboplastin reagent (HAEMOSIL) and an excess of calcium chloride (25mM) at 370C and calculating the time from addition of calcium till the formation of a fibrin clot. APTT was measured by incubating Platelet Poor Plasma at 370C with phospholipid (cephalin) and a contact activator (kaolin) followed by calcium and noting the time required for clot formation. PT and APTT were considered to be prolonged above the control values. Fibrinogen levels were measured by clauss assay. Normal reference range for fibrinogen was taken as-233-496mg/dl. D-dimers were measured by latex agglutination test on a glass slide. Commercial kits containing D-dimer monoclonal antibodies coupled to latex beads (0.25ml) is mixed with the test plasma (0.1ml) and agglutination was read macroscopically after 3 min. The result was recorded as either positive (elevated) or negative (normal). Factor VIII levels were measured using Factor VIII deficient plasma. Normal range of Factor VIII levels was taken as 50-150%. Bcr-abl was detected in the peripheral smear using PCR based technique (quantitative). With the help of clinical and laboratory information, patients were categorized into phase of the CML using the WHO criteria [1]. Patients were further assessed for the response to the treatment in the form of clinical, haematological and molecular remission according to the national comprehensive cancer network (NCCN) guidelines version 3.2014 [1]. Data was analysed using software SPSS version 20 for quantitative data. Associations were made using students t-test and correlations were drawn using spearman’s rank co-efficient. A p-value <0.05 was taken as significant.
Results
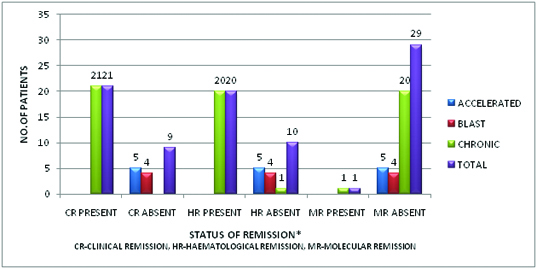
The study included 30 patients, out of which 17 were male and 13 were females (M:F ratio of 1.3:1) with a mean age of 35.53 ± 8.92 years. Mean duration of imatinib exposure in the study group was 15.5 ± 16.14 months, with the mean dose of imatinib being 460 ± 130.25 mg. Out of the 30 patients, 21(70%) were in chronic phase, 5(16.7%) were in accelerated phase and 4(13.3%) were in blast phase of the CML. Out of the 30 patients, 21(70%) were in clinical remission, 20(66.7%) were in haematological remission and 1(3.3%) was in molecular remission. The relationship of patients in various phases of disease with the phases of remission has been depicted in [Table/Fig-1]. The results of the correlation between haemostatic parameters (Platelet counts, PT, APTT, D-dimers, fibrinogen, Factor VIII levels) and phase of the disease and status of the remission using spearman’s co-efficient were as follows-
Relationship of various phases of the CML with the status of the remission (n=30).
Out of 30 patients, 9 (30%) patients had low platelet counts, 4 (13.3%) patients had high platelet counts and remaining 17 (56.7%) patients had normal counts. The distribution of number of patients with low, normal and high platelet counts according to phase of disease and status of remission has been shown in [Table/Fig-2]. No significant correlations were found between platelet counts and phase of the disease or status of remission. Out of the 30 patients, 4 (13.3%) patients had prolonged PT and 26 (86.7%) patients had normal PT. The distribution of the number of patients with prolonged and normal PT according to the phases of disease and remission status has been shown in the [Table/Fig-3]. We found a significant positive correlation between PT and phase of the CML (p-0.002) and a significant negative correlation between PT and clinical and haematological remission (p-0.003 and 0.006). Out of the 30 patients in the study, 9 (30%) had prolonged APTT and 21 (70%) had normal APTT values. The distribution of number of patients with prolonged and normal APTT according to the phase of disease and status of remission has been summarized in [Table/Fig-4]. There was no correlation between APTT and phase of the disease as well as status of remission. Eighteen patients (60%) patients in the study had positive results for D-dimers and 12 (40%) patients had normal (negative) results. The number of patients with positive results for D-dimers and their distribution according to disease phase and remission status has been tabulated [Table/Fig-5]. There was a significant positive correlation between D-dimers and phase (p-0.050) and a negative correlation between D-dimers and clinical and haematological remission (p-0.035 and 0.017). In our study, we found fibrinogen levels to be low in 10 (33%) patients and normal in 20 (67%) patients. The data of number of patients with low and normal fibrinogen levels according to phase of disease and remission status has been summarized in [Table/Fig-6]. We found a significant positive correlation between fibrinogen levels and phase (p-0.011) and a negative correlation between fibrinogen levels and clinical and haematological remission (p-0.010 and 0.005). Factor VIII levels were low in 3 (10%), high in 10 (33%) and normal in remaining 17 (56.7%) patients. The data of Factor VIII levels according to disease phase and remission status has been shown in [Table/Fig-7]. We found a significant positive correlation between the Factor VIII and phase (p-0.006) and a negative correlation between Factor VIII and the clinical and haematological remission (p-0.005 and 0.001).
Platelets according to phases and status of remission (n=30).
| Platelets (n) | Total(n) | Correlation coefficient and p-value |
|---|
| Low | Normal | High |
|---|
| Phase | Chronic | 6 | 15 | 0 | 21 | 0.219p-value-0.245 |
| Accelerated | 2 | 1 | 2 | 5 |
| Blast | 1 | 1 | 2 | 4 |
| Clinical | Yes | 6 | 15 | 0 | 21 | -0.241p-value-0.199 |
| No | 3 | 2 | 4 | 9 |
| Haematological | Yes | 5 | 15 | 0 | 20 | -0.138p-value-0.487 |
| No | 4 | 2 | 4 | 10 |
| Molecular | Yes | 0 | 1 | 0 | 1 | 0.080p-value-0.751 |
| No | 9 | 16 | 4 | 29 |
| Total | 9 | 17 | 4 | 30 | |
Prothrombin Time in various phases of CML and status of remission (n=30).
| Prothrombin time (n) | Total (n) | Correlation coefficient and p-value |
|---|
| Normal | Prolonged |
|---|
| Phase | Chronic | 20 | 1 | 21 | 0.547p-value-0.002 |
| Accelerated | 3 | 2 | 5 |
| Blast | 3 | 1 | 4 |
| Clinical | Yes | 20 | 1 | 21 | -0.526p-value-0.003 |
| No | 6 | 3 | 9 |
| Haematological | Yes | 19 | 1 | 20 | -0.490p-value-0.006 |
| No | 7 | 3 | 10 |
| Molecular | Yes | 1 | 0 | 1 | 0.161p-value-0.395 |
| No | 25 | 4 | 29 |
| Total | 26 | 4 | 30 | |
Activated Partial Thromboplastin Time in various phases of CML and status of the remission (n=30).
| APTT (n) | Total (n) | Correlation coefficient and p-value |
|---|
| Normal | Prolonged |
|---|
| Phase | Chronic | 16 | 5 | 21 | -0.099p-value-0.603 |
| Accelerated | 2 | 3 | 5 |
| Blast | 3 | 1 | 4 |
| Clinical | Yes | 16 | 5 | 21 | -0.080p-value-0.675 |
| No | 5 | 4 | 9 |
| Haematological | Yes | 16 | 4 | 20 | -0.139p-value-0.464 |
| No | 5 | 5 | 10 |
| Molecular | Yes | 1 | 0 | 1 | -0.032p-value-0.866 |
| No | 20 | 9 | 29 |
| Total | 21 | 9 | 30 | |
D-dimers according to phases and status of remission (n=30).
| D-dimers (n) | Total (n) | Correlation coefficient and p-value |
|---|
| Negative | Positive |
|---|
| Phase | Chronic | 11 | 10 | 21 | 0.361p-value-0.050 |
| Accelerated | 1 | 4 | 5 |
| Blast | 0 | 4 | 4 |
| Clinical | Yes | 11 | 10 | 21 | -0.386p-value-0.035 |
| No | 1 | 8 | 9 |
| Haematological | Yes | 11 | 9 | 20 | -0.433p-value-0.017 |
| No | 1 | 9 | 10 |
| Molecular | Yes | 0 | 1 | 1 | 0.152p-value-0.424 |
| No | 12 | 17 | 29 |
| Total | 12 | 18 | 30 | |
Fibrinogen according to phase and status of remission (n=30).
| Fibrinogen (n) | Total (n) | Correlation coefficient and p-value |
|---|
| Normal | Low |
|---|
| Phase | Chronic | 11 | 10 | 21 | 0.456p-value-0.011 |
| Accelerated | 5 | 0 | 5 |
| Blast | 4 | 0 | 4 |
| Clinical | Yes | 11 | 1 | 21 | -0.483p-value-0.010 |
| No | 9 | 0 | 9 |
| Haematological | Yes | 10 | 10 | 20 | -0.500p-value-0.005 |
| No | 10 | 0 | 10 |
| Molecular | Yes | 0 | 1 | 1 | -0.283p-value-0.161 |
| No | 20 | 9 | 29 |
| Total | 20 | 10 | 30 | |
Factor VIII levels according to phase and status of remission (n=30).
| Coagulation Factor VIII (n) | Total(n) | Correlation coefficient and p-value |
|---|
| Low | Normal | High |
|---|
| Phase | Chronic | 2 | 16 | 3 | 21 | 0.488p-value-0.006 |
| Accelerated | 1 | 0 | 4 | 5 |
| Blast | 0 | 1 | 3 | 4 |
| Clinical | Yes | 2 | 16 | 3 | 21 | -0.504,p-value-0.005 |
| No | 1 | 1 | 7 | 9 |
| Haematological | Yes | 2 | 16 | 2 | 20 | -0.582p-value-0.001 |
| No | 1 | 1 | 8 | 10 |
| Molecular | Yes | 0 | 1 | 0 | 1 | -0.085p-value-0.655 |
| No | 3 | 16 | 10 | 29 |
| Total | 3 | 17 | 10 | 30 | |
Discussion
We studied 30 patients of CML already on imatinib and assayed the haemostatic parameters in them and correlated them with the phase of the disease and their status of remission. M: F ratio in our study was 1.3:1. Literature reveals that in CML, males are affected more often than females (ratio of 1.3–2.2 to 1). Our results were in accordance with the reported ratio in the literature. The incidence of CML increases with age. The median age at presentation reported from the literature is 45–55 years [6–8]. However, in our study, the median age of the population was 35.53 ± 8.92 years. Our study population had a lower mean age, which was due to an unexplained geographical bias.
Platelets were low in 30% of the patients and high in 13.3% of the study group. A high platelet count contributes to thrombo-haemorrhagic complications in these patients. Since, all patients suffering from thrombo-haemorrhagic complications have thrombocytosis; it is considered a risk factor for haemostatic disorders [2]. Thrombocytosis may lead to vascular phenomenon is suggested by the evidence that platelet count reduction lowers the risk of microcirculatory disturbance [9–12]. Also, results from clinical studies suggest that cytoreductive therapy reduces not only the platelet counts but also the risk of thrombosis [13]. Studies have shown that Dasatinib causes impairement of platelet aggregation in response to epinephrine, collagen and bleeding may be the result of inhibition of Platelet derived growth factor receptor (PDGFR) Kinase [14,15]. Considering this effect of dasatinib on haemostasis; we excluded patients taking dasatinib from our study. High platelet counts can lead to bleeding complications by causing acquired von Villibrand’s disease [16]. Mechanisms proposed for this occurrence includes selective adsorption of High Molecular Weight Multimers (HMWM) on the surface of platelets resulting in absence of large multimers from the circulation [17], accelerated proteolysis of the multimeric forms of Von Willibrand’s Factor (VWF) by the action of various enzymes secreted by cells associated with myeloproliferative disorders [16] and development of auto-antibodies against VWF leading to its removal by reticulo-endothelial system [17].
Out of the 3 patients in the study who had elevated platelet counts, 2 had prolonged APTT also. This could be due to acquired von willibrand’s disease seen in association with thrombocytosis. Low platelets in our study might be due to the myelosuppressive effects of imatinib or due to acceleration of the disease itself. High platelets in the study (despite imatinib treatment) were the result of acceleration of the disease or blast phase. None of the patients in the study had clinical events of bleeding or thrombosis. This finding is in accordance with the previous observations that the haemostatic disturbances and clinically significant thrombo-haemorrhagic events are least common in the CML amongst all myeloproliferative neoplasm. Since progression of the phase of CML is associated with both a fall as well as rise in the platelet counts, hence no significant correlation was obtained in the study between the phase and the platelet counts.
We found that, PT was prolonged in 13.3% of the patients. Evica et al., found that PT was prolonged in 52% of CML patients [18]. Mansour et al., further found levels of Factor VII to be significantly low in patients with CML as compared to the control group, and concluded that reduced levels of Factor VII may account for prolonged PT seen in these patients [19]. Hasegawa et al., similarly studied 8 patients of CML and found prolonged PT in 1 patient (who had reduced Factor VII, in blast phase) [20]. However, we did not assayed Factor levels other than the Factor VIII. We found lower frequency of PT prolongation in our study. This might be an effect of imatinib on the Factor levels (mainly VII) and imatinib treatment might reduce the coagulation abnormality of extrinsic pathway seen in CML. There was a significant positive correlation (p-0.002) in the study between prolonged PT and the phase of the disease, suggesting a significantly more derangement of coagulation pathway with the progression of the CML phase. There was also a significant negative correlation between the PT and the clinical (p-0.003) and haematological (p-0.006) remission. This suggests that with the achievement of clinical and haematological remission, there was an improvement in the coagulation abnormalities of the extrinsic pathway.
We found that APTT was prolonged in 30% of the patients and there was no statistically significant correlation between the APTT prolongation and the phase of the disease and the status of remission. Evica et al., found that APTT was prolonged in 27.45% of CML patients [18]. Our results were almost in accordance with those previously reported in the literature. This might suggest that imatinib treatment had no significant effect on the intrinsic coagulation pathway. APTT can be prolonged in CML patients due to deficiency of Factor VIII (which is usually secondary to acquired von Villibrand’s disease), deficiency of Factor IX, Factor XII or Factor XI. Hasegawa et al., studied 8 patients of CML and reported APTT prolongation in 5 of them [20]. Cause of APTT prolongation was reduced Factor XII and IX in the first patient, reduced Factor XI in second, reduced Factor XI and XII in third and fourth and reduced Factor V in the fifth patient. Out of the 9 patients in our study who had prolonged APTT, 2 also had low Factor VIII levels and the cause of prolonged APTT in others is not known as we did not assay other coagulation factors. APTT prolongation was not found to be correlated with the phase of disease or remission status, meaning that the coagulation disturbance of the intrinsic pathway persists across all the phases of disease and during all the remissions.
We found that the D-dimers were positive (elevated) in 60% of the patients. D-dimers elevation is a marker of activation of the fibrinolytic pathway. D-dimers elevation was more marked in patients with hyperleucostasis due to the micro-occlusion of capillaries by the leukemic cells and tissue hypoxia, endothelial damage and resultant activation of both coagulation and fibrinolytic cascades. Ahmed et al., found that D-dimers are elevated in 78.6% of the patients with hyperleucocytic syndrome and none of the patients in their study without the hyperleucocytic syndrome had elevated D-dimers [4]. Four patients in our study had TLC above 100 X 109, but none had clinical features of hyperleucocytic syndrome. There was a significant positive correlation between D-dimers and phase of CML (p-0.050). This indicates that progression of CML is associated with significantly elevated D-dimers. Similarly there was a significant negative correlation between D-dimers and clinical (p-0.035) and haematological remission (p-0.017). This means that treatment with imatinib leading to achievement of clinical and haematological remission was associated with a significant normalisation of D-dimers.
We found fibrinogen levels to be low in 33.3% of the patients. This was in sharp contrast to the previously reported studies, which found elevated levels of fibrinogen in patients of CML In a study by Evica et al., it was found that fibrinogen levels are elevated in 36.27% of the CML patients [18] and Abegunde et al., concluded that elevated fibrinogen levels acts as a risk factor for thrombosis in these patients [21]. This finding might be an effect of treatment with imatinib on fibrinogen levels and may enable us to hypothesize that imatinib can lower the risk of clinical thrombosis in CML patients due to its fibrinogen lowering effect, although this finding needs further validation in similar studies in the future. There was a significant positive correlation between the fibrinogen levels and the phase of CML in the study (p-0.011). This means that with the progression of the phase of CML, there was a statistically significant increase of the fibrinogen levels despite imatinib treatment (indicating imatinib resistance). This finding can only be explained by the fact that all the patients in the study were on imatinib treatment and hence, imatinib treatment had a fibrinogen lowering effect in the study during the chronic phase. But, with the progression of disease phase despite imatinib, there was a significant elevation of fibrinogen levels (indicating imatinib resistance). Similarly, there was a statistically significant negative correlation between the fibrinogen levels and clinical (p-0.010) and haematological remission (p-0.005). Further studies with a larger sample size are needed in order to validate the above findings.
Ten percent of the patients in our study were found to have low Factor VIII levels and 33.3% had high Factor VIII levels. Although, low Factor VIII levels in CML can be explained on the basis of acquired von Villibrand’s disease seen in association with thrombocytosis, none of the 3 patients in our study with low Factor VIII levels had thrombocytosis. This finding indicates a deficiency of Factor VIII seen in CML, independent of acquired von-willibrand’s disease. This is an interesting finding as literature does not provide reported incidence of Factor VIII deficiency in CML as an isolated finding without the associated thrombocytosis. Hasegawa et al., studied 8 patients with CML and found Factor VIII levels to be high in 4 of them (50%), taking normal range to be 70-130%, and found that the coagulation abnormalities in their study did not revert to normal after chemotherapy, but reverted to normal after bone marrow transplant [20]. We found elevated Factor VIII levels in 33.3% of the patients, taking laboratory standard range to be 50-150%. Hence, our findings seem to be concordance with the previously reported findings of Hasegawa et al., (considering the different normal reference range) [20]. We also found a statistically significant positive correlation between Factor VIII levels and the phase of CML (p-0.006) indicating a significant increase of Factor VIII levels as the disease progresses. Similarly there was a statistically significant negative correlation between the Factor VIII levels and the clinical (p-0.005) and haematological remission (p-0.001). This finding indicates that there was significant lowering of Factor VIII levels with the achievement of clinical and haematological remission due to imatinib treatment. We did not find any statistically significant correlation between any of the haemostatic parameter and molecular remission, a finding likely due to the fact that only one patient in our study had achieved complete molecular remission.
Conclusion
The findings need validation in further studies with a larger sample size. We found that haemostatic system was significantly deranged in patients of CML. The study tried to explain the observed findings on the basis of the existing literature and also found some interesting findings previously undescribed which included low fibrinogen levels in patients (in chronic phase, on imatinib) and isolated low levels of Factor VIII. This is probably the first study of its kind assessing these parameters in various phases of CML as well as the response of imatinib treatment on them.
Consent and conflicts of interests: The authors declare no conflicts of interests. Proper written and informed consent was taken from the patients prior to inclusion in the study.