Cisplatin is one of the most commonly used chemotherapeutic agents in the treatment of various solid malignancies including head and neck cancer and cancer of the uterine cervix. Results of meta-analysis in head and neck and cervical cancer have shown that chemoradiotherapy with cisplatin based chemotherapy is superior to radiotherapy alone for locoregional control of malignancies [1]. The commonly used schedules of cisplatin in this clinical situation are 1) 30-40 mg/m2 given as a short infusion of one hour or 2) 100 mg/m2 given as a three hour infusion. Cisplatin acts as a radiosensitizer when given concurrently with radiotherapy [2]. For the synergistic action of cisplatin as a radiosensitizer the time interval between the infusion of cisplatin and delivery of radiotherapy is important. Treatment protocols which correlated with the pharmacokinetic and radiobiological properties of the components used may result in improved outcome [3]. The aim of the study was to characterize the free and total concentration of cisplatin after long and short duration infusion which will help to address two clinical issues: 1) What are the total and free concentrations of cisplatin post infusion of the above two cisplatin regimens; 2) Assuming that the radiosensitizing property of cisplatin occurs with higher plasma concentrations of cisplatin, we aim to predict the optimum time over which radiation therapy can be administered following the infusion protocol.
Materials and Methods
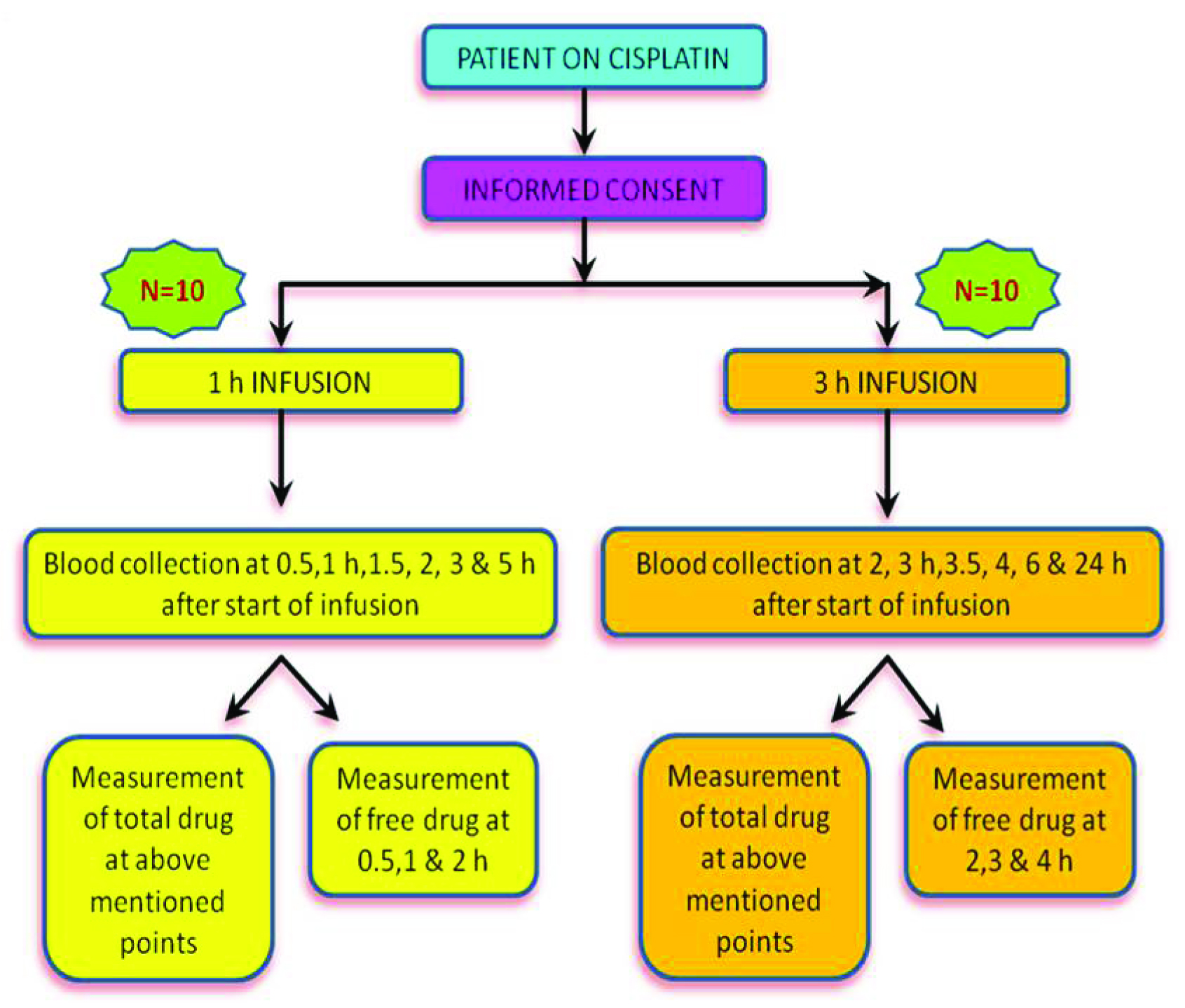
Patients and Treatment Protocol
This is a double arm, prospective, non randomized exploratory pilot study conducted between November 2012 to November 2013 (13 months). Twenty adult patients diagnosed with squamous cell cancer of either head and neck region or uterine cervix were prospectively included for the study. All patients were on treatment with concurrent chemoradiotherapy with cisplatin. Patients were recruited after obtaining written informed consent following GCP/ICH guidelines [4]. The study was approved by the Institutional Review Board. These groups of patients received either of the two schedules of cisplatin 1) 40 mg/m2 as 1 hour infusion weekly, 2) 100 mg/m2 3 hour infusion 3 weekly [5]. The dose and regimen was decided by the treating radiation oncologist, based on the Eastern Cooperative Oncology Group (ECOG) performance status, clinical diagnosis and stage of the disease [6]. Patients were treated with megavoltage teletherapy machine (12 patients in Linear accelerator and 8 patients in telecobalt machine).
Materials and Chromatographic Conditions
Cis-diamminedichloroplatinum(II) (CDDP), Sodium-Diethyldithiocarbamate (DDTC) and Nickel Chloride (used as internal standard) were purchased from Sigma Aldrich, India Ltd. For the analysis the column used was a C18–Discovery Supelcosil™ (Analytical products Sigma Aldrich), 250 x 4.6 mm(5 μm), which was maintained at a temperature of 30°C. Detection was by UV at 254nm. The mobile phase was Methanol: Acetonitrile: Water at 40:30:30 [7] at pH 3.7 and a flow rate of 1.2 mL.min-1. Total run time for the assay was 26 min. Amicon® Ultra (Merck Millipore®) 0.5mL filters were used to collect the plasma ultrafiltrate.
Blood Collection and Sample Preparation
In both groups, blood sampling was performed during the first cycle of treatment. On the day of cisplatin infusion, blood samples for the measurement of cisplatin concentration were collected at defined time points. An IV cannula for blood collection was inserted into a forearm vein in the arm opposite to that used for the cisplatin infusion.
Blood samples (4ml each) were collected into EDTA tubes at 0.5, 1, 1.5, 2, 3 and 5 hours from the start of infusion in the 1 hour infusion group and 2, 3, 3.5, 4, 6 and 24 hours from the start of the infusion in the 3 hour infusion group. Blood samples were transported in polystyrene boxes with ice packs and protected from light by wrapping them in aluminium foil [8,9]. Upon arrival in the laboratory, plasma was immediately separated in a dark room by centrifugation at 13000 × g for 8 min at 4°C and stored in the dark at -70°C till analysis [10].
Free cisplatin was measured only in plasma collected at 0.5, 1, and 2 hour from the start of infusion in the 1 hour infusion regimen group and at 2, 3 and 4 hour from the start of infusion in the 3 hour infusion regimen group. To obtain the ultrafiltrate, 500 μl of the plasma samples was loaded into Centrifree® cartridges and centrifuged at 13000 × g for 30 min at 4°C. The ultrafiltrate was stored at -70oC, until analysis for free cisplatin concentration.
Sample Extraction: The stock solution of cisplatin was prepared in 0.9% NaCl [11]. Further dilutions were made in 0.9% NaCl which were used to make the following calibrators in plasma (0.5, 1.0, 2.5, 7.5, 10 and 20 μg/mL respectively). For the measurement of free concentration of cisplatin, calibrators (0.5, 1.0, 2.5, 7.5 and 10 μg/mL) were prepared in 0.9% NaCl. 10% DDTC used as a chelating agent, was prepared in 0.1N NaOH and vortexed until completely dissolved. For the estimation of total and free cisplatin concentration, 150μl or 100μl Nickel chloride respectively was mixed with 100 μl of 10% DDTC in a microcentrifuge tube and made up to 1 ml with MilliQ® water. Then it was vortexed well for 30 sec and preincubated for 30 min at 37°C in a water bath, prior to the extraction procedure [10].
For the derivatization extraction method to estimate the concentration of total cisplatin, 200 μl of plasma sample was mixed with 40 μl of preincubated nickel chloride (150 μg/ml) and 20 μl of 10% DDTC, to form DDTC2 Pt complex [12]. The above mixture was vortexed for 1 min and incubated at 37°C in a water bath for exactly 60 min [14]. 450 μl of ethyl acetate was then added and vortexed for a minute. After centrifugation at 13000x g for 8 min at 4°C, 300 μl of supernatant organic phase was removed immediately and concentrated by drying. After which it was reconstituted with 100 μl of 50% methanol and 20 μl was injected into the system.
For estimation of free cisplatin concentration, 100 μl of plasma ultrafiltrate was mixed with 20 μl of pre-incubated Nickel chloride (100 μg/ml) and 10 μl of 10% DDTC. The above mixture was vortexed gently for 15 seconds and incubated at 37°C in a water bath for exactly 60 min. After which, 20 μl was injected into the system.
Statistical Analysis
All statistical calculations were performed using SPSS version 20.0 (IBM). Log transformed total and free concentration of cisplatin in each of the infusion groups were checked for assumptions of repeated measures analysis of variance (rANOVA). Mauchly’s test of sphericity was performed prior to performing rANOVA. Pairwise comparisons for different time points in each of the infusion groups were performed by using Bonferroni correction only if the overall test was significant. Descriptive statistics were presented as Geometric mean (Gmean) (with 95% Confidence Interval (CI)) for each time point.
Results
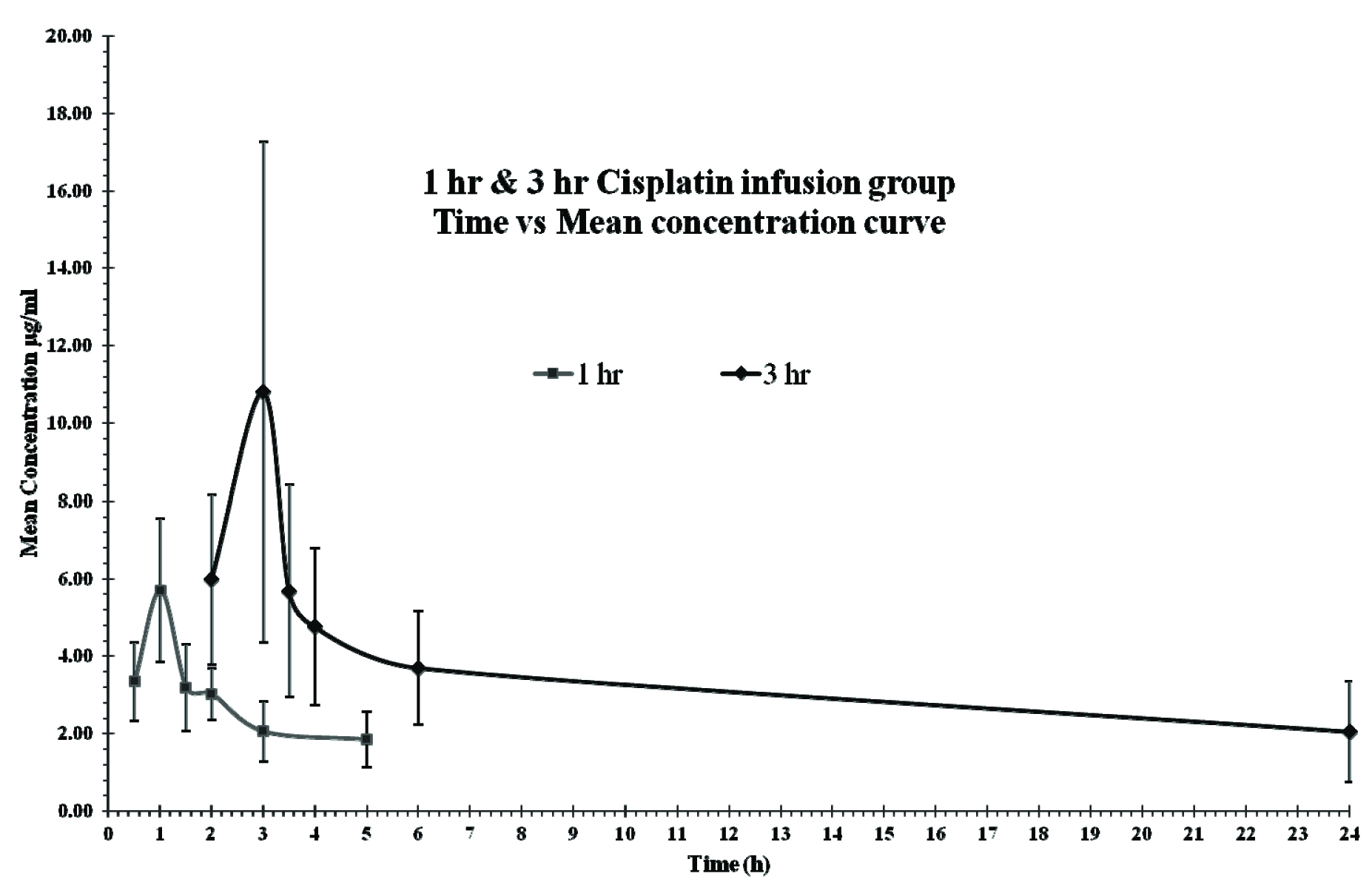
Total 20 adult patients (16 males and 4 females) were recruited into the study, 10 each in the 1 hour and 3 hour infusion regimen group [Table/Fig-1]. Baseline demographic characteristics are given in [Table/Fig-2]. It is apparent from the table that, even though it was a non-randomized study the two groups were comparable for important renal and haematological parameters. Plasma concentration time profiles of both regimens are shown in [Table/Fig-3].
Demographic characteristics
| S. No. | Parameters | 1 hr infusion group Mean (SD) | 3 hr infusion group Mean (SD) | p-value |
|---|
| 1 | Age(Yrs) | 44.6(7.7) | 43.1(9.7) | 0.37 |
| 2 | Weight(Kg) | 55.95(10.9) | 58.46(7.6) | 0.27 |
| 3 | Height(Cm) | 161.1(9.9) | 165.4(7.2) | 0.05 |
| 4 | BSA(M2) | 1.577(0.2) | 1.639(0.1) | 0.06 |
| 6 | Hb(g/L) | 11.64(1.5) | 11.56(0.9) | 0.43 |
| 7 | Total Count | 8330.0(2568.6) | 6870.0(2412.5) | 0.09 |
| 8 | Platelet Count | 239700.0 (104238.0) | 208500.0 (87805.7) | 0.25 |
| 9 | Creatinine(mg/dl) | 0.96(0.2) | 1.054(0.2) | 0.13 |
| 10 | Cr Cl(ml/min) | 77.3(22.9) | 72.7(12.8) | 0.33 |
| 11 | ANC (Absolute Neutrophil count) | 5379.7(1977.6) | 3926.0(1471.5) | 0.05 |
| 12 | Albumin g/dl | 4.4(0.6) | 4.2(0.5) | 0.30 |
Mean concentration time profiles of 1 hr and 3hr infusion regimens for cisplatin.
Platinum Concentration Decline Pattern with Time One Hour Infusion Group
The Gmean (±sd) Cmax of total cisplatin was 5.37(±1.47) μg/ml with a Tmax at the end of infusion (1 h from start of infusion). The mean (±sd) AUC0.5-5 was 12.5 (±3.7) (range:7.61-19.4) mg.h/L. Interpatient variability in Cmax and AUC0.5-5, measured as coefficient of variation was 32.5% and 29.5% respectively. With Tmax at 1 hour, comparison of Cmax with concentrations in the plasma in later time points (after 1 hour up to 5 hour post infusion) was performed using rANOVA. There was a significant difference between concentration at 1 hour and 1.5 hour (p-value=0.002), between 1 hour and 2 hour (p-value=0.007), 1 hour and 3 hour (p-value=0.000) and 1 hour and 5 hour (p-value=0.000). However, there was no significant difference between concentration at 1.5 hour and 2 hour (p-value = 1.00). Although when compared to 3 hour, there was a significant difference with the concentration at 1.5 hour (p value= 0.02). This is shown in [Table/Fig-3,4].
Time vs Gmean concentration(total) in 1 hour infusion group.
| Time (h) | GMean | G SD | 95% Confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| 0.5 | 3.23 | 1.32 | 2.66 | 3.94 |
| 1 | 5.37 | 1.47 | 4.07 | 7.09 |
| 1.5 | 3.03 | 1.39 | 2.39 | 3.84 |
| 2 | 2.95 | 1.25 | 2.51 | 3.47 |
| 3 | 1.92 | 1.47 | 1.46 | 2.54 |
| 5 | 1.73 | 1.46 | 1.32 | 2.27 |
The mean percentage decline at 1.5, 2, 3 and 5 hour in comparison to the Cmax at 1 hour, was 41.43%, 41.94%, 65.45% and 74.77% respectively
With respect to concentration of free drug, Gmean (±sd) concentration at 0.5, 1 and 2 hour was 1.2(±2.95), 2.25(±2.05) and 0.48(±2.69) μg/ml respectively. rANOVA test showed a significant difference in the free drug concentration at 2 hour compared to 1 hour (p-value= 0.000). This resulted in a 73.9% mean decline in free drug concentration by 2 hour compared to the end of the infusion. This is shown in [Table/Fig-5].
Time vs Gmean concentration(Free) in 1 hour infusion group.
| Time(h) | GMean | G SD | 95% Confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| 0.5 | 1.20 | 2.95 | 0.55 | 2.60 |
| 1 | 2.25 | 2.05 | 1.35 | 3.76 |
| 2 | 0.48 | 2.69 | 0.24 | 0.97 |
The Gmean free fraction (as %) showed maximum free fraction at the end of infusion which decreased at 2 hour after the start of infusion. However in this regimen, a significant difference was obtained by rANOVA test between the free fraction (%) at 1 hour and 2 hour (p-value= 0.002).
Three Hour Infusion group
The geometric mean (±sd) Cmax of total cisplatin concentration was 9.03(±1.93) μg/ml with a tmax at the end of infusion (3 hour from start of infusion). The mean (±sd) AUC2-24 in this group was 75.5 (29.9) (range: 38.7- 128.1) mg.h/L. Interpatient variability in Cmax and AUC2-24 measured as coefficient of variation was 59.7% and 39.6% respectively.
A comparison of Cmax with concentrations in the plasma from later time points (up to 24 hour post infusion) was performed using rANOVA. The test showed a significant difference between concentrations at 3 and 3.5 hours (p- value=0.041), between 3 hour and 4 hour (p-value=0.008), 3 hour and 6 hour (p-value=0.002) and 3 hour and 24hour (p-value=0.002). There was no significant difference between concentrations at 3.5 and 4 hours (p-value= 1.00). However, when compared to 6 hour there was a significant difference with the concentration at 3.5 hour (p-value= 0.016) This is shown in [Table/Fig-3,6].
Time vs Gmean concentration(total) in 3 hour infusion group.
| Time(h) | GMean | G SD | 95% Confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| 2 | 5.59 | 1.49 | 4.20 | 7.43 |
| 3 | 9.03 | 1.93 | 5.65 | 14.45 |
| 3.5 | 5.11 | 1.64 | 3.59 | 7.27 |
| 4 | 4.33 | 1.63 | 3.06 | 6.14 |
| 6 | 3.47 | 1.46 | 2.65 | 4.54 |
| 24 | 1.66 | 2.13 | 0.97 | 2.85 |
The mean percentage decline at 3.5, 4, 6 and 24 hour in comparison to the Cmax at 3 hour, was 39.02%, 44.02%, 58.02% and 75.95% respectively.
With respect to the concentration of free drug, Gmean (±sd) concentration at 2, 3 and 4 hr was 0.63(±2.14), 1.5(±2.36) and 0.4(±1.75) μg/ml. rANOVA showed a significant difference in the free drug concentration at 4 hour compared to 3 hour (p-value= 0.000). There was a 70.8% mean percentage decline in free drug concentration by 4 hour compared to the end of infusion. This is shown in [Table/Fig-7].
Time vs Gmean concentration (Free) in 3 hour infusion group.
| Time(h) | GMean | G SD | 95% Confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| 2 | 0.63 | 2.14 | 0.37 | 1.09 |
| 3 | 1.50 | 2.36 | 0.81 | 2.78 |
| 4 | 0.40 | 1.75 | 0.26 | 0.59 |
No significant difference was found by rANOVA between the free fraction at 3.0 hour and 4.0 hour (p-value = 0.6).
Poor correlation (r) was observed between serum albumin and total cisplatin concentration at any time point, in both the regimens (r = 0.28 to - 0.36 at any measured time point).
Discussion
In our study, the highest concentration of total and free cisplatin was achieved at the end of the infusion in both the regimens. Our finding is in agreement with that reported by Ikeda and Urien [13,14]. Begg AC showed that higher doses of cisplatin led to the formation of increased DNA adducts [15]. This could signify that radiation if given at the time of maximum or higher concentration of cisplatin, may help to achieve maximum benefit.
Marcu et al., suggested from an in vitro study that a shorter time sequence between cisplatin and concomitant radiotherapy led to improved tumour control. They also reported that cisplatin when administered daily along with radiation led to a 35% improvement of tumour control as compared to a regimen of weekly one dose of cisplatin with daily radiation [16]. Schwachofer et al., also stated that this beneficial effect of cisplatin as a radiosensitizer may not hold if there is a time gap of 24 hours between cisplatin and radiation [17].
If radiation is to be administered when cisplatin (total and free) concentrations are at their maximum, then it would mean administering the radiotherapy immediately on cessation of the IV infusion. This would mean that the IV infusion and the pre-arrangements for radiation therapy would need to happen simultaneously which may have inherent practical difficulties.
In this study, the total cisplatin geometric mean concentration declined at 30 minutes after the end of infusion in the 1 hour and in 3 hour infusion regimen by 41.4% and 39% respectively, compared to the end of infusion. Therefore the best time to administer radiation, if it is not possible at the end of infusion, would be within 30 minutes after the end of infusion. In the event of a delay of 30 minutes or more, our findings suggest that for both regimens there is no significant difference in the total cisplatin concentration between that at 30 minutes and 1 hour after the end of infusion. This is consistent with the findings of Gorodetsky et al., who had shown that the supra-additive effect of cisplatin and radiation is only achieved when the drug is administered before or shortly after radiation therapy. The possible explanation for this could be alteration of the cellular oxygenation, cross resistance or redistribution in cell cycle [18].
Cisplatin has 90% plasma albumin binding [8], however the difference in protein binding during and after the infusion did not influence the tmax of free cisplatin (based on the time points for total and free cisplatin in our study). In the 1 hour regimen, the mean free platinum concentration declined by 73.9% one hour after the end of infusion in comparison to the decline in the total drug concentration of 41.9%. A similar pattern is seen in the 3 hour regimen where the mean free platinum concentration declined by 70.8 % one hour after the end of the infusion and the total drug concentration declined by 44.0%. The rapid decline of the free drug concentration compared to the total cisplatin concentration is in agreement with the finding reported by Urien S et al., [14]. However, since the free drug concentration was not measured earlier than 1 hour after end of infusion, it is not possible to comment on any earlier decline in the free drug concentration.
Cisplatin is mainly cleared by renal elimination. All the patients included in this study had creatinine clearance within acceptable limits; this may explain the rapid clearance of cisplatin within the first hour after the end of the infusion.
As cisplatin is highly photosensitive and the chelation of cisplatin with DDTC during extraction is highly time and temperature dependent [19] immense care had to be taken from the time of sampling and during extraction to preserve the accuracy, precision and robustness of the assay.
A limitation of the study is the number of time points in the first hour after the end of the infusion. This limitation made it difficult to comment on the rate of decline within the first 30 minutes after the end of the infusion. It would be useful to include multiple time points in the first hour post-infusion, this would enable us to categorically define the exact time until when the cisplatin concentration (total and free) would remain within acceptable limits. A case report by C Charlier et al., had quoted a possible therapeutic range for cisplatin was 1.0-5.0 μg/mL [20]. It needs to be emphasized however that to our knowledge an acceptable concentration range in which to administer radiation therapy has not yet been clearly defined. A second limitation is the small sample size, however in view of cisplatin being widely used as a concurrent chemotherapy agent with radiotherapy, this data is still relevant.
Conclusion
In conclusion, with regard to the radiosensitizing property of cisplatin, the ideal time to administer radiation therapy would be immediately at the end of the cisplatin infusion, in both regimens. If this is not possible for practical reasons, the delay should not exceed 30 minutes after the end of infusion. In centres where even this is not possible, we have estimated through the measurement of total cisplatin concentration that there is no significant decline in concentration achieved at 30 minutes and one hour post infusion. However, it is important to note that there is a highly significant reduction in free cisplatin concentration one hour after the end of infusion.