Introduction
Epilepsy affects 65 million people worldwide and entails a major burden in seizure-related disability, mortality, co morbidities, stigma, and costs [1].
Epilepsy is a disease of the brain and is defined by any of the following conditions: (a) At least two unprovoked seizures occurring > 24 hours apart; (b) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60 %) after two unprovoked seizures, occurring over the next 10 years; (c) diagnosis of an epilepsy syndrome [2].
Seizure is an event and is defined by transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain. Seizures may be either focal or generalized. Focal seizure begin focally in a cortical site, can be further described as having motor, sensory, autonomic, cognitive or other features. Generalized seizures are characterized by widespread involvement of bilateral cortical regions at the onset. They are usually accompanied by impairment of consciousness. They can further be divided into absence, tonic-clonic, clonic, tonic, atonic and myoclonic seizure types [3].
Seizure with a known cause (brain tumour, stroke, head injury, meningitis, encephalitis, neurocysticercosis, genetic syndromes etc.,) is called secondary seizure. Idiopathic (primary) seizure has no identifiable cause. It is genetically determined and affects otherwise normal people of both sexes.
Idiopathic Generalized Tonic-Clonic Seizure (GTCS) is the most common seizure type. In the initial tonic phase patients experience contraction of muscles throughout the body and body stiffening. After few seconds it is followed by clonic phase of rhythmic jerking of the face and limbs which is produced by the superimposition of periods of muscle relaxation on the tonic muscle contraction. EEG during tonic & clonic phases shows characteristic features of GTCS.
First-line antiepileptic drugs (AEDs) for GTCS are valproic acid, lamotrigine and topiramate. Alternatives are zonisamide, phenytoin, carbazepine, oxcarbazepine, phenobarbital, primidone and felbamate [4].
Valproic Acid (VPA) and Lamotrigine (LTG) are sodium ion channel modulators. They enhance fast inactivation of the sodium ion channels and as consequences of this action they block action potential prolongation, stabilize neuronal membranes and diminish neurotransmitter release, focal firing and seizure spread [5].
VPA and LTG also block calcium ion channels. They reduce the flow of calcium ions through calcium channels and as a consequence of this action they diminish neurotransmitter release (N & P types), diminish slow depolarization (T type) and reduce spike wave discharges.
LTG enhances HCN channel activity and thus buffers large hyperpolarization and depolarization inputs and suppresses action potential initiation by dendritic inputs. In addition to this it also acts upon the postsynaptic neuronal membrane via suppression of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors and reduces glutamate release in granule cells of dentate gyrus [6].
A significant proportion of patients with generalized epilepsy need to switch from their initial drug to second drug in order to achieve seizure control. It has been suggested that lamotrigine may be used as an alternative to valproic acid if valproic acid is poorly tolerated or contraindicated [7].
The present study was carried out to compare the effectiveness of lamotrigine and valproic acid as first-line monotherapy in newly diagnosed idiopathic generalized tonic-clonic seizures in adults.
Materials and Methods
The present prospective randomized comparative study was conducted in the Department of Pharmacology in collaboration with Department of Medicine at Teerthanker Mahaveer Medical College & Research Centre (TMMC&RC), Moradabad, Uttar Pradesh, during the period from April 2014 to March 2015, on adult patients suffering from idiopathic generalized tonic-clonic seizure (GTCS). Patients were included in the study group on the basis of inclusion and exclusion criteria. This study was approved by Institutional Ethics Committee. A written informed consent was obtained from each patient of the study group.
Inclusion criteria
(a) Age 18 years or above, (b) Newly diagnosed idiopathic generalized tonic-clonic seizures, (c) Patient had experienced clearly observable minimum of two or more generalized tonic-clonic seizures in the preceding one year, (d) Normal general and neurological findings on clinical examination, (e) Normal features in computed tomography (CT Scan) / magnetic resonance imaging (MRI) of brain, (f) Normal intelligence.
Exclusion criteria
(a) Secondary generalized tonic–clonic seizures; (b) Progressive neurological disorder; (c) A psychiatric disorder requiring medication; (d) Clinically significant chronic hepatic, renal and cardiac conditions; (e) Any disease that could interfere with drug absorption, distribution, metabolism, or excretion; (f) Long term co-medication with other drugs; (g) Suspected poor compliance; (h) Pregnant or lactating women; (i) Had been treated with investigational antiepileptic drugs in previous one year.
A detailed history was obtained from patients, relatives or witnesses about the pattern of seizures in Out Patient Department/Indoor Patient Department of Medicine, TMMC&RC. Seizure type was based on the clinical information available.
All the patients underwent general, neurological and other systemic examinations. Investigations included complete blood count, plasma glucose, serum alanine aminotransferase, serum aspartate aminotransferase, serum gamma–glutamyltransferase, blood urea, serum creatinine, CT / MRI imaging of brain, tests for HIV I & II, urinalysis, EEG. All 60 patients were divided into two equal number groups in a random fashion with the help of table of random numbers. Randomization was coordinated separately within the two centers in TMMC&RC, and the identity of allocated treatment was not masked. VPA group patients (n=30) received 200 mg/300mg/500mg enteric-coated non-sustained release sodium valproate tablet. LTG group patients (n=30) received lamotrigine 25mg / 50mg /100mg dispersible non-sustained release tablet.
Valproic acid was initiated at 10 mg/kg/day in two to three divided doses. Dose was increased by 5 mg/kg/day every 3 days until seizures were controlled, intolerable side effects occurred, or a maximum dose of 30 mg/kg/day had been reached. Lamotrigine was initiated at 0.5 mg/kg/day in two divided doses for 2 weeks, followed by 1.0 mg/kg/day for additional two weeks. Thereafter, dose was increased by 1 mg/kg/day until seizures were controlled, intolerable adverse effects occurred, or a maximum dose of 12 mg/kg/day had been reached.
Each patient was advised to visit in the medicine outpatient department of TMMC & RC at monthly interval for 12 months follow-up. At each visit, the evaluation included questioning of the patients or relatives (external observers) about presence or absence of clinical features of generalized tonic-clonic seizures and adverse drug reactions. Medical examination was also done and baseline laboratory investigations (haematological and biochemical) were repeated at the visit. Contact with patients was also maintained through telephone/mobile calls.
Effectiveness was assessed by reduction from baseline in the percentage of patients remaining seizure-free and by reduction from baseline in the mean number of seizures per month, after 12 months follow-up. For this purpose all patients were provided with diaries to record regularly the number and time of occurrence of seizure. If the patient remained seizure-free, ‘none’ was recorded in the diary every day. Patients were also instructed to record adverse event in the seizure diary.
The control of seizure was classified as followed:
(a) Excellent control: seizure–free.
(b) Good control: >/ = 50% reduction in seizure frequency from baseline.
(c) Poor control: <50% reduction in seizure frequency from baseline.
Adverse effects were analyzed by Naranjo Adverse Drug Reaction Probabilty Scale and causality assessment was done in every adverse effect reported. Each adverse drug reaction was assigned to a probability category from the total score as follows: definite if the overall score was 9 or greater, probable for a score of 5 to 8, possible for 1 to 4 and doubtful if the score was 0 [8].
Statistical Analysis
Data was obtained in mean, standard deviation and number (n) and percentage (%) and was analysed by unpaired student t-test. The significance was determined by using Chi-square test. All data were analysed with statistical software SPSS version 21.0.
Results
All 60 patients enrolled for the study had newly diagnosed idiopathic generalized tonic-clonic seizures. Age, sex and duration of epilepsy were comparable in both valproic acid and lamotrigine study groups.
The study group included 37 males and 23 females, ranging in age from 18 to 70 years [Table/Fig-1,2].
Age distribution in VPA (n=30) and LTG (n=30) groups.
| Age group (years) | VPA group | LTG group |
|---|
| n | (%) | n | (%) |
|---|
| 18-20 | 8 | (26.67) | 5 | (16.67) |
| 21-30 | 6 | (20.00) | 8 | (26.67) |
| 31-40 | 6 | (20.00) | 9 | (30.00) |
| 41-50 | 6 | (20.00) | 5 | (16.67) |
| 51-60 | 3 | (10.00) | 2 | (6.66) |
| 61-70 | 1 | (3.33) | 1 | (3.33) |
| Total | 30 | (100.00) | 30 | (100.00) |
n = number, (%) = percentage, VPA group = valproic acid group, LTG group = lamotrigine group.
Gender distribution in VPA (n=30) and LTG (n=30) groups.
| Gender | VPA group | LTG group |
|---|
| n | (%) | n | (%) |
|---|
| Male | 20 | (66.67) | 17 | (56.67) |
| Female | 10 | (33.33) | 13 | (43.33) |
n = number, (%) = percentage, VPA group = valproic acid group, LTG group = lamotrigine group.
After three months of treatment, 16 (53.33%) patients taking valproic acid and 8 (26.67%) patients taking lamotrigine group were seizure-free. At six months, seizure freedom was observed in 19 (63.33%) patients taking valproic acid and 14 (46.67%) patients taking lamotrigine. At the last observation after 12 months followup, 23 (76.67%) patients taking valproic acid and 17 (56.67%) patients taking lamotrigine were seizure-free. This difference was statistically significant (p < 0.03) [Table/Fig-3,4].
Treatment response in VPA group (n=30).
| After treatment | Effectiveness of valproic acid |
|---|
| ExcellentControl | GoodControl | PoorControl |
|---|
| n | % | n | % | n | % |
|---|
| 3 months | 16 | (53.33) | 3 | (10.00) | 11 | (36.67) |
| 6 months | 19 | (63.33) | 3 | (10.00) | 08 | (26.67) |
| 12 months | 23 | (76.67) | 1 | (03.33) | 06 | (20.00) |
n = number, (%) = percentage.
Response to treatment in LTG group (n=30).
| After treatment | Effectiveness of lamotrigine |
|---|
| ExcellentControl | GoodControl | PoorControl |
|---|
| n | % | n | % | n | % |
|---|
| 3 months | 8 | (26.67) | 2 | (06.66) | 17 | (56.67) |
| 6 months | 14 | (46.67) | 3 | (10.00) | 10 | (33.33) |
| 12 months | 17 | (56.67) | 3 | (10.00) | 7 | (23.33) |
n = number, (%) = percentage.
3 patients withdrew from study, 27 patients remained in the trial.
In valproic acid group, mean seizure frequency at baseline was 5.17 per month and after 12 months of treatment it decreased to 1.70 per month. In lamotrigine group patients, mean seizure frequency at baseline was 4.93 per month and after 12 months of treatment it decreased to 2.43 per month. Statistical analysis revealed significant difference (p < 0.001) [Table/Fig-5].
Seizure frequency per month at baseline and after 12 months treatment.
| Number of seizures(per month) | VPA group | LTG group |
|---|
| Mean (SD) | Mean (SD) |
|---|
| Baseline | 5.17 (2.00) | 4.93 (1.74) |
| After 12 months treatment | 1.70 (1.82) | 2.43 (1.87) |
SD = Standard Deviation, VPA group = valproic acid group, LTG group = lamotrigine group.
Adverse effects were recorded in 9 (30.00%) patients of valproic acid group and 17 (56.66%) in lamotrigine group patients. Sedation, ataxia and tremor were recorded in patients taking valproic acid but these symptoms responded to a decrease in dosage. Skin rash developed within three months in 3 (10.00%) patients taking lamotrigine, they withdrew from the study [Table/Fig-6]. Flow chart of the study participants is presented in [Table/Fig-7].
Adverse effects in VPA (n=30) and LTG (n=30) groups.
| Adverse effects (ADR) | VPA group | LTG group | Causality relationship | AED continued/ stopped |
|---|
| n | (%) | n | (%) |
|---|
| Gastrointestinal symptoms | 3 | (10.00) | 3 | (10.00) | Possible | Continued |
| Headache | 0 | | 3 | (10.00) | Possible | Continued |
| Skin rash | 0 | | 3 | (10.00) | Probable | Stopped |
| Sedation | 2 | (6.66) | 1 | (3.33) | Possible | Continued |
| Ataxia | 2 | (6.66) | 0 | | Possible | Continued |
| Tremor | 2 | (6.66) | 0 | | Possible | Continued |
| Insomnia | 0 | | 1 | (3.33) | Possible | Continued |
| Cough | 0 | | 3 | (10.00) | Possible | Continued |
| Decreased appetite | 0 | | 1 | (3.33) | Possible | Continued |
| Dizziness | 0 | | 1 | (3.33) | Possible | Continued |
| Total | 9 | (30.00) | 17 | (56. 66) | |
n = number, (%) = percentage, VPA group = valproic acid group, LTG group = lamotrigine group, Causal relationship = causal relationship between ADR and treatment, Causal relationship possible = naranjo scale total score 1 to 4, Causal relationship probable = naranjo scale total score 5 to 8, AED = antiepileptic drug.
Flow chart of the study participants.
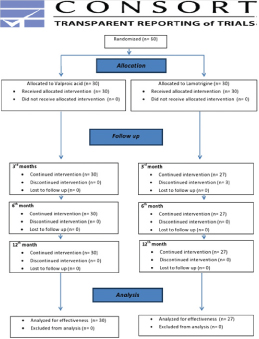
Discussion
The present prospective randomized comparative study evaluated the effectiveness of valproic acid and lamotrigine as first-line drug in the treatment of newly diagnosed idiopathic generalized tonic-clonic seizures in adults. The valproic acid was noticed to have better effectiveness profile than lamotrigine. A total of 76.67% patients treated with valproic acid and 56.67% patients treated with lamotrigine became seizure free after 12 months follow-up.
The present study findings are consistent with studies conducted by Steinhoff et al., Marson et al., Nicolson et al., and Mazukiewicz-Beldzinska et al., who have also reported valproic acid superiority over lamotrigine as first-line monotherapy in newly diagnosed idiopathic generalized tonic-clonic seizures in adults and is inconsistent with Stephen et al., study which has reported valproic acid as well as lamotrigine monotherapy to have equal efficacy in the same group of patients [9–13]. Many non-comparative studies have also reported effectiveness of valproic acid as well as lamotrigine monotherapy in newly diagnosed idiopathic generalized tonic-clonic seizures in adults [14–18].
The seizure- free rate observed in the present study (76.67%) in valproic acid group was higher than Nicolson et al., (52.10%), Stephen et al., (47%), Coppola et al., (70%), Trinka et al., (64.50%) and lower than Steinhoff et al., (83.30%) [9,11,13,14,17]. The seizure free rate in lamotrigine group in the present study (56.67%) was higher than Nicolson et al., (16.70%), Stephen et al., (47%) and lower than Steinhoff et al., (60.60 %), Coppola et al., (60%), Steinbaugh et al., (66.50%), Yamamoto et al., (80%), Rosenow et al., (64.50%) [9,11,13–16,18].
In the present study, adverse effects were recorded in 30% cases in valproic acid group and 56.66% cases in lamotrigine group. The commonest adverse effects noticed were gastrointestinal symptoms, headache and skin rash in lamotrigine group and gastrointestinal symptoms, sedation, ataxia and tremor in valproic acid group. In valproic acid group, they were tolerable and did not require withdrawal of drugs but three patients of lamotrigine group withdrew because of dermatological adverse effects.
The incidence and pattern of adverse effects observed in the present study were consistent with previous studies except Yamamoto et al., study which reported higher (82% cases) adverse effects in lamotrigine group [16]. Withdrawal of lamotrigine due to dermatological responses has also been reported by Marson et al., and Mazurkiewicz-Beldzinska et al., [9,12].
The results of the present study are significant, in the context using the drugs on Indian patients. Confounding variable in the present study was social class.
Limitation
The limitations of present study were that serum levels of valproic acid and lamotrigine were not measured due to lack of facilities, patients number were limited and follow-up was done for one year only.
Conclusion
Both valproic acid and lamotrigine are effective as first-line monotherapy for the treatment of newly diagnosed adults with idiopathic generalized tonic-clonic seizures but valproic acid is preferable to lamotrigine because of being more effective and safe. Long term studies with large population are needed for further evaluation of these drugs.
n = number, (%) = percentage, VPA group = valproic acid group, LTG group = lamotrigine group.
n = number, (%) = percentage, VPA group = valproic acid group, LTG group = lamotrigine group.
n = number, (%) = percentage.
n = number, (%) = percentage.
3 patients withdrew from study, 27 patients remained in the trial.
SD = Standard Deviation, VPA group = valproic acid group, LTG group = lamotrigine group.
n = number, (%) = percentage, VPA group = valproic acid group, LTG group = lamotrigine group, Causal relationship = causal relationship between ADR and treatment, Causal relationship possible = naranjo scale total score 1 to 4, Causal relationship probable = naranjo scale total score 5 to 8, AED = antiepileptic drug.
[1]. Moshe SL, Perucca E, Ryvlin P, Tomson T, Epilepsy new advancesThe Lancet 2015 385:884-98. [Google Scholar]
[2]. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, ILAE Official Report: a practical clinical definition of epilepsyEpilepsia 2014 55:475-82. [Google Scholar]
[3]. Lowenstein DH, Seizure and Epilepsy. In :Longo DL, Fauci AS, Kasper AS, Hauser SL, Jameson JL, Loscaizo JL, edsHarrison’s Principles of Internal Medicine 2012 18th edMcGraw-Hill Companies, Inc:3251-65. [Google Scholar]
[4]. Rosati A, Masi SD, Guerrini R, Antiepileptic drug treatment in children with epilepsyCNS Drug 2015 29:847-63. [Google Scholar]
[5]. McNanara JO, Pharmatherapy of the epilepsies. In: Bruton L, Chabner B, Knollman B, edsGoodman & Gilman’s The Pharmacological Basis of Therapeutics 2012 12th edMcGraw-Hill Companies Inc:543-600. [Google Scholar]
[6]. Lee CY, Fu WM, Chen CC, Su MJ, Liou HH, Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrusEpilepsia 2008 49:888-97. [Google Scholar]
[7]. Kaski D, Cockerell C, Advances in the Diagnosis and management of epilepsy in adultsPrescriber 2015 26:12-19. [Google Scholar]
[8]. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, A method for estimating the probability of adverse drug reactionsClin Pharmacol Ther 1981 30:239-45. [Google Scholar]
[9]. Steinhoff B J, Uberall M A, Seimes H, Kurlemann G, Schmitz B, Bergmann L, The LAM-SAFE Study : Lamotrigine versus carbamazepine or valproic acid in newly diagnosed focal and generalised epilepsies in adolescents and adultsSeizure 2005 14:597-605. [Google Scholar]
[10]. Marson AG, Al-Kharusi AM, Alwaidh M, The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trialLancet 2007 369:1016-26. [Google Scholar]
[11]. Nicolson A, Appleton RE, Chadwick DW, Smith DF, The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsiesJ Neurol Neurosurg Psychiatry 2004 75:75-79. [Google Scholar]
[12]. Mazurkiewicz-Beldzinska M, Szmuda M, Matheisel A, Long-term efficacy of valproate versus lamotrigine in treatment of idiopathic generalized epilepsies in children and adolescentsSeizure 2010 19:195-97. [Google Scholar]
[13]. Stephen LJ, Sills GJ, Leach JP, Butler E, Parker P, Hitiris N, Sodium valproate versus lamotrigine; a randomised comparison of efficacy, tolerabiltity and effects on circulating androgenic hormones in newly epilepsyEpilepsy Res 2007 75(2-3):122-29. [Google Scholar]
[14]. Coppola G, Auricchio G, Federico R, Carotenuto M, Pascotto A, Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical seizures : an open-lebel, randomized, parallel-group studyEpilepsia 2004 45:1049-53. [Google Scholar]
[15]. Steinbaugh L, Szaflarski JP, Adjunctive therapy for the treatment of primary generalized tonic-clonic seizures : focus on once daily lamotrigineDrug Des Devel Ther 2010 4:337-42. [Google Scholar]
[16]. Yamamoto T, Hong SB, Shimizu M, Sato K, Numachi Y, Lamotrigine monotherapy in newly diagnosed epilepsy or recurrent epilepsy : A multi-center, open–lebel studyEpilepsy & Seizure 2014 7:55-65. [Google Scholar]
[17]. Trinka E, Marson AG, Van Paesschen W, Kälviäinen R, Marovac J, Duncan B, KOMET: an unblinded, randomised, two parallel-group, stratified trial comparing the effectiveness of levetiracetam with controlled-release carbamazepine and extended-release sodium valproate as monotherapy in patients with newly diagnosed epilepsyJ Neurol Neurosurg Psychiatry 2013 84:1138-47. [Google Scholar]
[18]. Rosenow F, Schade-Brittinger C, Burchardi S, Klein KM, Weber Y, The Lali Mo Trial: Lamotrigine compared with levetiracetam in the initial 26 weeks of monotherapy for focal and generalized epilepsy – open lebel, prospective, randomized controlled multicentric studyJ NeurolNeurosurg Psychiatry 2012 83:1093-98. [Google Scholar]