Minor Salivary Gland Changes in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma - A Histopathological Study
Sunil Paramel Mohan1, Ravi Teja Chitturi2, Yoithapprabhunath Thukanayakanpalayam Ragunathan3, Suman Jhansi Lakshmi4, Jaisanghar Nallusamy5, Isaac Joseph6
1 Head of Department, Deparment of Oral Pathology, Director, Department of Stem Cells and Regenerative Medicine, Dean, Sree Anjaneya Institute of Dental Sciences, Calicut, Kerala, India.
2 Lecturer, Department of Oral Biology, School of Dentistry, Faculty of Medical Sciences, The University of the West Indies, St. Augustine Campus, Trinidad and Tobago.
3 Reader, Department of Oral and Maxillofacial Pathology, Vivekanandha Dental College for Women, Tamilnadu, India.
4 Professor, Department of Oral Medicine and Radiology, K S R Institute of Dental Science and Research, Tiruchengode, Tamilnadu, India.
5 Professor, Department of Oral Medicine and Radiology, Sree Anjaneya Institute of Dental Sciences, Calicut, Kerala, India.
6 Professor and Head, Department of Oral Pathology, Sree Moogambigai Dental College, Kulasekharam, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sunil Paramel Mohan, Head of Department, Deparment of Oral Pathology, Director, Department of Stem Cells and Regenerative Medicine, Dean, Sree Anjaneya Institute of Dental Sciences, Calicut-673323, Kerala, India.
E-mail: sunilnarien@gmail.com
Introduction
The most common etiology for Oral Squamous Cell Carcinoma (OSCC) is tobacco and tobacco related products which cause nuclear damage to the keratinocytes. The chemical carcinogens not only affect the lining of oral epithelium but also affect the lining epithelium of the excretory ducts of the salivary glands. Thus, there is a possibility of epithelial dysplasia of the salivary duct epithelium which may lead to potential malignant transformation.
Aim
The study was performed to see the changes in the minor salivary glands and excretory ducts in cases of oral epithelial dysplasia and OSCC.
Materials and Methods
A total of 278 archival cases of mild, moderate and severe epithelial dysplasia, carcinoma in situ, OSCC including verrucous carcinoma were histopathologically evaluated to observe changes in the excretory ducts and the minor salivary glands.
Results
In the study there were 56.5% males and 43.5% females. The age group that was most commonly affected in both the sexes was 50-60 yr old. Buccal mucosa was the most common site of involvement. Ductal changes observed in the excretory duct include simple hyperplasia, metaplastic changes such as mucous, oncocytic & squamous, and infiltration of inflammatory cells and malignant cells. Acinar changes observed were degeneration, squamous metaplasia, myoepithelial cell proliferation and inflammatory cell infiltration. Both the excretory ducts and ducts within the gland showed dysplasia.
Conclusion
According to observations in our study it is suggested that histopathological interpretation for oral mucosal lesions especially oral epithelial dysplasias and OSCC should also include changes related to salivary gland tissue to provide a better treatment plan and prevent recurrence of the malignant tumours.
Acinar changes, Excretory duct, Hyperplasia, Metaplasia, Salivary gland tumour
Introduction
The history of cancer is as old as our world. The history goes back to 2500 BC when cancer was described in the Hindu epic “The Ramayana” [1]. Oral cancer or OSCC is one of the leading causes of death in India. The most common etiology for OSCC is tobacco and tobacco related products [2]. These chemical carcinogens cause nuclear damage in the keratinocytes and ultimately lead to epithelial dysplasia and OSCC. The chemical carcinogens not only affect the lining of oral epithelium but also affect the lining epithelium of the excretory ducts of the salivary glands. Thus, there is a possibility of epithelial dysplasia of the salivary duct epithelium which may lead to potential malignant transformation which in some studies has been shown to be around 1.45%. Although the spread of epithelial dysplasia along glandular ducts in uterine cervical epithelial neoplasia is a well-recognized phenomenon, its relation to the excretory ducts and minor salivary glands by carcinogenic stimuli is not well documented. The purpose of this study was to analyze the frequency of salivary gland involvement and their changes in routine tissue sections of oral epithelial dysplasias and carcinomas. We recorded the histological changes present in the ductal epithelium and salivary glands and correlated them to epithelial dysplasia and OSCC.
Materials and Methods
A total of 278 hematoxylin and eosin stained archival slides from Tamil Nadu Government Dental College, Chennai which included cases of mild, moderate and severe epithelial dysplasia, carcinoma in situ, OSCC were histopathologically evaluated for the study after approval from the ethics committee. Of these, those with presence of salivary gland tissue were included in the study. The age, sex, site and histopathological diagnosis were collected from the records. The slides which exhibited ductal and glandular involvement were selected for the study. The total number of oral epithelial dysplasias and OSCC of different grades, verrucous carcinoma and carcinoma in situ were recorded. Oral epithelial dysplasia was graded as; mild, moderate and severe and OSCC was graded as well, moderately and poorly differentiated. The ductal changes in the salivary gland were recorded as ductal proliferation, metaplasia, dysplasia, inflammatory cell infiltration and malignant squamous infiltration. The acinar changes in the minor salivary glands were recorded as degeneration, squamous metaplasia, myoepithelial cell proliferation and inflammatory cell infiltration. The results were tabulated and statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software v.17.0 (IBM, USA) for Windows 7 (Microsoft, USA).
Results
Demographic data: The distribution of cases taken for the study has been shown in [Table/Fig-1]. Among the males, 6 (4%) showed lip involvement, 46 (31.7%) cases were in the buccal mucosa, 9(6.2%) in the palate, 16 (11%) in the tongue, 13 (6.9%) in the floor of mouth, 38 (26.2%) in the alveolus, 5 (3.4%) in the maxillary antrum and 12 (8.3%) cases in the retromolar area. In females 3 (2.3%) showed lip involvement, 70 (52.6%) were in the buccal mucosa, 5 (3.8%) in the palate, 8 (6%) in the tongue, 2 (15%) in the floor of mouth, 39 (29.3%) in the alveolus, 3 (2.3%) in the maxillary antrum and the retromolar area. [Table/Fig-2] shows the age distribution of oral epithelial dysplasias and OSCC in males and females. Maximum number of cases were observed in 51-60 years age group.
Demographic details of cases taken for the study.
| Sex | Epithelial Dysplasias | CIS | Percent | VC | OSCC | Percent | Total |
|---|
| D1 | D2 | D3 | WD | MD | PD |
|---|
| Males | 9 | 1 | 3 | - | 56.5 | 2 | 56 | 64 | 10 | 52 | 145 |
| Females | 5 | 3 | 2 | 1 | 43.5 | 2 | 49 | 63 | 8 | 48 | 133 |
| Total | 14 | 4 | 5 | 1 | - | 4 | 105 | 127 | 18 | - | 278 |
D1 – Mild dysplasia, D2- Moderate dysplasia, D3 – Severe Dysplasia, CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, WD – well differentiated, MD – Moderately differentiated, PD – Poorly differentiated.
Age distribution of cases taken for the study.
| Oral Lesions | 10-20 yrs | 21-30 yrs | 31-40 yrs | 41-50 yrs | 51-60 yrs | 61-70 yrs | 71-80 yrs | 81-90 yrs |
|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F |
|---|
| OSCC | 1 | - | 3 | - | 7 | 8 | 21 | 21 | 41 | 40 | 37 | 33 | 19 | 15 | 1 | 3 |
| ED | 1 | - | 1 | - | - | 1 | 5 | 2 | 1 | 3 | 4 | 3 | 1 | - | - | - |
| VC | - | - | - | - | - | - | - | - | 1 | - | - | 2 | 1 | - | - | - |
| CIS | - | - | - | - | - | - | - | 1 | - | - | - | 1 | - | - | - | - |
| Total | 2 | - | 4 | - | 7 | 9 | 26 | 23 | 43 | 43 | 41 | 39 | 21 | 15 | 1 | 3 |
| Percent | 1.4 | 0 | 2.8 | 0 | 4.8 | 6.8 | 18 | 17.3 | 29.7 | 32.3 | 28.3 | 29.3 | 14.5 | 11.3 | 0.7 | 2.3 |
CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, ED – Epithelial dysplasia.
Ductal changes: The ductal changes observed in the excretory duct in various oral lesions have been listed in [Table/Fig-3]. The excretory duct seen in cases of OSCC showed eight cases of simple hyperplasia, six cases of squamous metaplasia and one case each of mucous and oncocytic metaplasia. Moderate dysplastic changes were seen in five cases and one case showed severe dysplasia. An inflammatory cell infiltrate was seen in 15 cases and seven cases showed infiltration by malignant cells into the duct. Ductal changes seen in the salivary gland were listed in [Table/Fig-4]. In OSCC we observed 15 cases of ductal proliferation and four cases of ductal metaplasia. Dysplastic changes of varying severity were observed in 13 cases which include nine cases of mild dysplasia and two cases each of moderate and severe dysplasia. Eighteen cases showed infiltration by malignant squamous cells.
Ductal changes in the excretory duct.
| Oral Lesions | Simple Hyperplasia | Metaplastic Hyperplasia | Dysplasia | Inflammatory Cells | Infiltration by Malignant Cells |
|---|
| M | O | S | Nil | + | ++ | +++ | Nil | + | ++ | +++ |
|---|
| OSCC | 8 | 1 | 1 | 6 | 3 | 6 | 5 | 1 | 1 | 12 | 2 | - | 7 |
| ED | - | - | 1 | - | - | - | - | - | - | - | - | - | - |
| VC | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CIS | - | - | - | - | - | - | - | - | - | - | - | - | - |
CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, M – Mucous, O – Oncocytic, S - Squamous, ED – Epithelial dysplasia, + - mild, ++ - moderate, +++ - severe.
Ductal changes in the salivary gland.
| Oral Lesions | Ductal Proliferation | Metaplasia | Dysplasia | Inflammatory Cells | Infiltration by Malignant Cells |
|---|
| Nil | + | ++ | +++ | Nil | + | ++ | +++ |
|---|
| OSCC | 15 | 4 | 16 | 9 | 2 | 2 | 3 | 11 | 13 | 2 | 18 |
| ED | 3 | 1 | - | - | - | - | - | - | - | - | - |
| VC | - | - | - | - | - | - | - | - | - | - | - |
| CIS | - | - | - | - | - | - | - | - | - | - | - |
CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, ED – Epithelial dysplasia, + - mild, ++ - moderate, +++ - severe
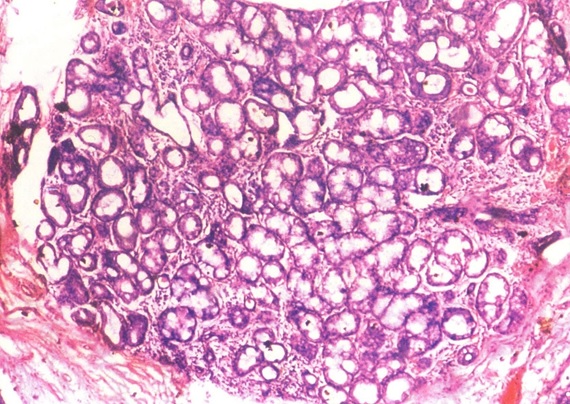
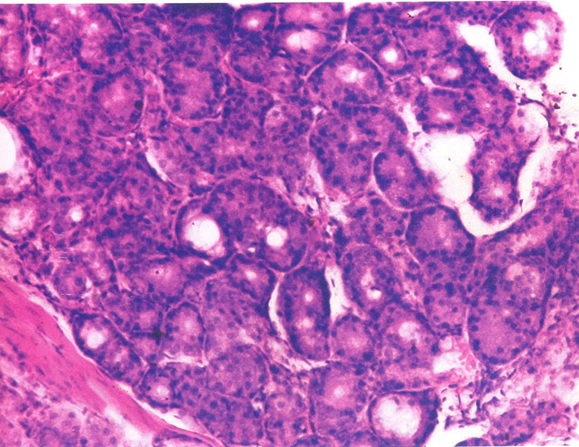
Acinar changes: [Table/Fig-5] shows the acinar changes observed in our study. In our study 29 cases showed acinar degeneration [Table/Fig-6], 20 cases showed squamous metaplasia [Table/Fig-7] and eight cases showed myoepithelial cell proliferation. Out of these 30 cases one case showed only proliferation, 11 cases showed acinar degeneration of mild and moderate intensity and 18 cases showed both ductal and acinar degeneration [Table/Fig-8]. Inflammatory cell reaction varied from mild inflammation in 11 cases, moderate inflammation in 16 cases and severe inflammation in three cases. [Table/Fig-9,10] shows dysplastic salivary gland cells in the connective tissue, dysplastic changes in epithelium, excretory duct and gland.
Acinar changes in the salivary gland.
| Degeneration | Squamous Metaplasia | Myoepithelial cell | Inflammatory Cells | Infiltration by Malignant Cells |
|---|
| Nil | + | ++ | +++ |
|---|
| 29 | 20 | 8 | 2 | 11 | 16 | 3 | 18 |
+ - mild, ++ - moderate, +++ - severe.
Photomicrograph of salivary gland showing acinar degeneration (H&E stain X40).
Photomicrograph of salivary gland showing squamous metaplasia (H&E stain X100).
Ductal proliferation and acinar degeneration in salivary glands.
| Normal | Without Gland | Only Proliferation | Only Degeneration | Both | Total |
|---|
| 2 | 1 | + | + = 9++ = 2 | 18 | |
| 2 | 1 | 1 | 11 | 18 | 33 |
+ - mild, ++ - moderate
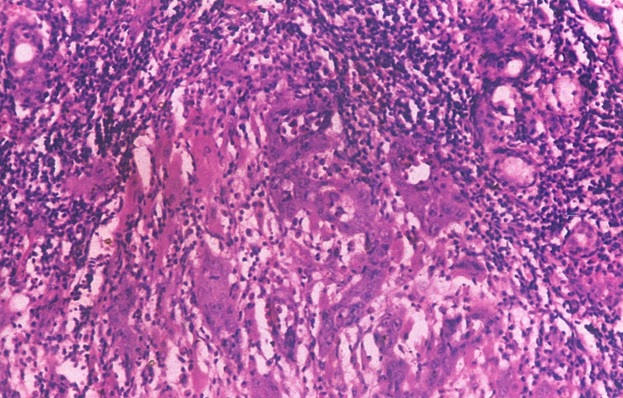
Photomicrograph showing streaming of dysplastic salivary gland cells in the connective tissue (H&E stain X100).
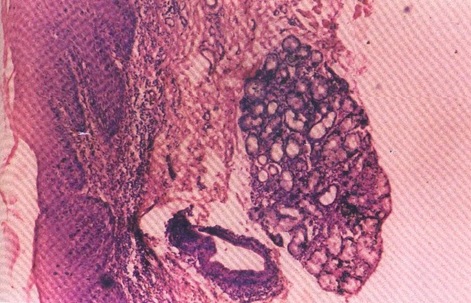
Photomicrograph showing dysplastic changes in epithelium, excretory duct and gland (H&E stain X40).
Discussion
One of the important causes for OSCC is tobacco and tobacco related products. Recently areca nut has also been proposed as an etiological factor for OSCC [3–5]. Although the etiology for Potentially Malignant Disorders (PMD) has been well defined, the cause for salivary gland carcinomas has not been that clear. Viruses such as Epstein Barr Virus (EBV) have been associated with malignant tumours of the salivary glands [6]. Association of ionizing radiation and risk of salivary gland tumours has been described by few authors [7,8]. Another important etiological factor associated with salivary gland cancers is occupation related. Exposure to metals in the plumbing and rubber industry, nickel compounds, woodworkers and personnel in the automobile industry are some of the occupations associated with increased risk of salivary gland cancers. Other occupations include personnel involved in asbestos mining and those in beauty shops and hairdressing [9ဓ12]. However the effect of epithelial dysplasia and OSCC on the minor salivary gland is not well documented. Hence, this study was undertaken to investigate the salivary glandular changes in reported cases of oral epithelial dysplasia and OSCC.
In this study the most common site where oral epithelial dysplasia and OSCC occurred were in the buccal mucosa and 50-60 years age group was most commonly affected as observed in other studies [13–15]. This is due to the tobacco chewing habit of the patients of both the sexes.
The different changes observed in the minor salivary glands along with ducts were carefully assessed. In the excretory duct following changes were observed which include; simple hyperplasia, mucous, oncocytic and squamous metaplasia and dysplasia. Simple hyperplasia is the increase in the thickness of same type of cells in the duct. The simple hyperplasia and metaplasia are adaptive changes that occur in the excretory duct possibly due to the effect of chemical carcinogens or due to OSCC [16,17]. Although these changes are reversible, their occurrence is highly undesirable and persistence of this adverse environment may predispose such tissues to dysplastic changes and a fertile area for malignant transformation. The presence of varying degrees of dysplasia was also observed in our study among cases of epithelial dysplasia, OSCC and verrucous carcinoma which is similar to the study by Browne RM and Potts AJC [18]. This dysplasia may be due to accumulation of the potential carcinogens over a length of time and it has been postulated that the degree of dysplasia in a duct increased with the increasing severity in the epithelium [19].
The glandular changes observed in our study were proliferation and degeneration of the salivary acini. These changes have been observed possibly due to several reasons. The chemical carcinogens might have caused ischemia which in turn might have induced proliferation and degeneration of salivary gland acini [20,21].
We observed squamous metaplasia of the acini in 20 out of 32 cases. Similar to ductal metaplasia, squamous changes in the acini can also be as a result of OSCC or irritation by chemical carcinogens and ischemia. It has been observed in experimental conditions that the squamous metaplasia can turn into squamous cell carcinoma via a non-genotoxic, proliferation-dependent mechanism [22]. It should also be noted that the acinar intercalated duct complex plays a very important role in development of salivary gland tumours. We also observed that 25% of our sections showed a myoepithelial cell proliferation (neoplastic myoepithelial cells) which is a very important constituent in the development of malignant tumours in the salivary glands [23–25]. About 90.6% of our cases showed presence of inflammatory cells in the salivary gland of varying intensities. The presence of inflammatory cells is indicative of immunological defense reaction of the host in contrast to metaplastic or dysplastic changes which favours malignant transformation [26].
In our study one case of severe dysplasia and a case of squamous cell carcinoma showed changes both in the surface epithelium and the salivary gland. The excretory duct of the oral dysplastic lesion showed ductal dysplasia within the glandular zone. The dysplastic glandular cells showed a streaming effect [Table/Fig-9]. This histological appearance suggests the possible histogenesis of adenosquamous carcinoma, the squamous cell carcinoma arising from the surface epithelium and adenocarcinoma arising from the excretory ductal and salivary glandular dysplastic epithelial cells. The simultaneous malignant changes in these two different types of epithelium were observed in our study [Table/Fig-10]. The probable origin is the surface epithelium with changes seen in the salivary gland [27,28].
It is very important to assess the changes in the minor salivary glands in cases of oral epithelial dysplasia and OSCC. Removal of salivary gland tissue is essential which may act as a potential source for recurrence of oral epithelial dysplasia and OSCC. Care should be taken to remove appropriate amount of tissue while excising pre-malignant and malignant lesions. The excised tissue should bear the minor salivary glands which have undergone changes during the process of dysplasia. There have been reports of recurrence of OSCC due to inadequate removal of salivary gland tissue and hence it is very important to remove the salivary tissue while excising oral epithelial dysplasias or OSCC [29–31].
Limitation
Though this is a preliminary study, further studies to evaluate why the changes occur in minor salivary glands is important.
Conclusion
The present study revealed interesting histopathological changes of salivary glandular tissue in cases of oral epithelial dysplasia and OSCC. In view of these observations it is suggested that histopathological interpretation for oral mucosal lesions especially oral epithelial dysplasia and OSCC also includes changes related to salivary gland tissue.
D1 – Mild dysplasia, D2- Moderate dysplasia, D3 – Severe Dysplasia, CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, WD – well differentiated, MD – Moderately differentiated, PD – Poorly differentiated.
CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, ED – Epithelial dysplasia.
CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, M – Mucous, O – Oncocytic, S - Squamous, ED – Epithelial dysplasia, + - mild, ++ - moderate, +++ - severe.
CIS – Carcinoma in situ, VC – Verrucous carcinoma, OSCC – Oral Squamous cell carcinoma, ED – Epithelial dysplasia, + - mild, ++ - moderate, +++ - severe
+ - mild, ++ - moderate, +++ - severe.
+ - mild, ++ - moderate
[1]. Pitot HC, In: Cancer yesterday and today. Pitot HC and Loeb DD editorsFundamentals of Oncology 2002 4th editionNew YorkMarcel Dekker Inc:25 [Google Scholar]
[2]. Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, Cancer mortality in India: a nationally representative surveyLancet 2012 379(9828):1807-16. [Google Scholar]
[3]. Prasad LK, Burden of oral cancer: an Indian scenarioJ Orofac Sci 2014 6:77 [Google Scholar]
[4]. Shah G, Chaturvedi P, Vaishampayan S, Arecanut as an emerging etiology of oral cancers in IndiaIndian J Med Paediatr Oncol 2012 33:71-79. [Google Scholar]
[5]. Warnakulasuriya S, Trivedy C, Peters TJ, Areca nut use: an independent risk factor for oral cancerBMJ 2002 324:799-800. [Google Scholar]
[6]. Wen S, Mizugaki Y, Shinozaki F, Takada K, Epstein-Barr virus (EBV) infection in salivary gland tumors: lytic EBV infection in nonmalignant epithelial cells surrounded by EBV-positive T-lymphoma cellsVirology 1997 227:484-87. [Google Scholar]
[7]. Spitz MR, Tilley BC, Batsakis JG, Gibeau JM, Newell GR, Risk factors for major salivary gland carcinoma: a case-comparison studyCancer 1984 54:1854-59. [Google Scholar]
[8]. Preston-Martin S, Thomas DC, White SC, Cohen D, Prior exposure to medical and dental x-rays related to tumors of the parotid glandJ Natl Cancer Inst 1988 80:943-49. [Google Scholar]
[9]. Horn-Ross PL, Britt-Marie L, Morrow M, Environmental Factors and the Risk of Salivary Gland CancerEpidemiology 1997 8:414-19. [Google Scholar]
[10]. Mancuso TF, Brennan MJ, Epidemiological considerations of cancer of the gallbladder, bile ducts and salivary glands in the rubber industryJ Occup Med 1970 12:333-41. [Google Scholar]
[11]. Swanson GM, Belle SH, Cancer morbidity among woodworkers in the U.S. automotive industryJ Occup Med 1982 24:315-19. [Google Scholar]
[12]. Milham S Jr, Cancer mortality patterns associated with exposure to metalsAnn NY Acad Sci 1976 271:243-49. [Google Scholar]
[13]. Pires FR, Ramos AB, Oliveira JB, Tavares AS, Luz PS, Santos TC, Oral squamous cell carcinoma: clinicopathological features from 346 cases from a single oral pathology service during an 8-year periodJ Appl Oral Sci 2013 21:460-67. [Google Scholar]
[14]. Yardimci G, Kutlubay Z, Engin B, Tuzun Y, Precancerous lesions of oral mucosaWorld J Clin Cases 2014 2:866-72. [Google Scholar]
[15]. Starzyńska A, Pawłowska A, Renkielska D, Michajłowski I, Sobjanek M, Błażewicz I, Oral premalignant lesions: epidemiological and clinical analysis in the northern Polish populationPostepy Dermatol Alergol 2014 31:341-50. [Google Scholar]
[16]. Dardick I, Jeans MT, Sinnott NM, Wittkuhn JF, Kahn HJ, Baumal R, Salivary gland components involved in the formation of squamous metaplasiaAm J Pathol 1985 119:33-43. [Google Scholar]
[17]. Raimondi AR, Vitale-Cross L, Amornphimoltham P, Gutkind JS, Molinolo A, Rapid development of salivary gland carcinomas upon conditional expression of K-ras driven by the cytokeratin 5 promoterAm J Pathol 2006 168:1654-65. [Google Scholar]
[18]. Browne RM, Potts AJ, Dysplasia in the salivary gland ducts in sublingual leukoplakia and erythroplakiaOral Surg Oral Med Oral Pathol 1986 62:44-48. [Google Scholar]
[19]. Daley TD, Lovas JG, Peters E, Wysocki GP, McGaw TW, Salivary gland duct involvement in oral epithelial dysplasia and squamous cell carcinomaOral Surg Oral Med Oral Pathol Oral Radiol Endod 1996 81:186-92. [Google Scholar]
[20]. Standish SM, Shafer WG, Serial histological effects of rat submaxillary and sublingual salivary gland duct and blood vessel ligationJ Dent Res 1957 36:866-79. [Google Scholar]
[21]. Vered M, Daniel N, Hirshberg A, Dayan D, Histomorphologic and morphometric changes in minor salivary glands of the rat tongue during 4-nitroquinoline-1-oxide-induced carcinogenesisOral Oncol 2003 39:491-96. [Google Scholar]
[22]. Takegawa K, Mitsumori K, Onodera H, Yasuhara K, Kitaura K, Shimo T, Induction of squamous cell carcinomas in the salivary glands of rats by potassium iodideJpn J Cancer Res 1998 89:105-09. [Google Scholar]
[23]. Batsakis JG, Kraemer B, Scuibba J, The pathology of head and neck tumours – The myoepithelial cell and its participation in salivary gland neoplasia – part IIHead and Neck Surg 1970 5:222-33. [Google Scholar]
[24]. Dardick F, Jeans MT, Sinnott NM, Wittkohn JF, Kahn HJ, Baumal R, Salivary gland components involved in the formation of squamous metaplasiaAm J Pathol 1985 119:33-43. [Google Scholar]
[25]. Chitturi RT, Veeravarmal V, Nirmal RM, Reddy BVR, Myoepithelial cells of the salivary glands in health and tumoursJ Clin Diagn Res 2015 9:ZE14-18. [Google Scholar]
[26]. Rakoff-Nahoum S, Why cancer and inflammation?Yale J Biol Med 2006 79(3-4):123-30. [Google Scholar]
[27]. Abdul Sayed RA, Sangulza OP, New House RF, Singh BS, Adenosquamous carcinoma – a case report with immunohistochemical evaluationOral Surg 1998 85:173-77. [Google Scholar]
[28]. Dardick I, Ho J, Paulus M, Mellon PL, Mirels L, Submandibular gland adenocarcinoma of intercalated duct origin in Smgb-Tag miceLab Invest 2000 80:1657-70. [Google Scholar]
[29]. Genden EM, Ferlito A, Silver CE, Takes RP, Suárez C, Owen RP, Contemporary management of cancer of the oral cavityEur Arch Otorhinolaryngol 2010 267:1001-17. [Google Scholar]
[30]. Chiesa F, Boracchi P, Tradati N, Rossi N, Costa L, Giardini R, Risk of preneoplastic and neoplastic events in operated oral leukoplakiasOral Eur J Cancer 1993 29:23-28. [Google Scholar]
[31]. Silverman S, Gorsky M, Lozada F, Oral leukoplakia and malignant transformation of a followup study of 257 patientsCancer 1984 53:563-68. [Google Scholar]