Nitric Oxide (NO) is a ubiquitous molecule required for multifarious physiological functions in all vertebrates, including neurovascular coupling in the mammalian brain [1,2]. Neurovascular coupling is on important function of NO because the human brain comprises merely 2%-3% of body weight but consumes 20%-25% of the oxygen supply [3]. NO ensures matching of the brain’s energy supply with neuronal activity, glutamatergic uptake by astrocytes, increased transport of glucose and delivery of lactate to neurons to protect against insufficient ATP for energizing neuronal signaling [4]. Dietary nitrate supplementation is a recently popularized method for increasing the blood level of NO, purported to decrease oxygen consumption, lessen muscle fatigue and improve athletic performance [5]. The blood pressure-lowering and haemodynamic effects of Beetroot Juice (BJ), a nitrate-rich beverage, is shown to decrease blood pressure in healthy individuals, but may not be useful as an adjunct to antihypertensive treatments in patients diagnosed with hypertension [6,7]. BJ-induced improvement in cognitive functions is reported to accompany improvement in cerebrovascular haemodynamics [8].
Augmentation Index (AIx), measured by sphygmomanography is reported to be an indicator of arterial stiffness, cardiovascular aging and risk for cardiovascular disease [9,10]. A modification of the AIx measurement technique used to correlate stiffness of the radial artery associated with aging has been applied for computation of CAIx [11]. This method has been used to demonstrate greater cerebral arterial stiffness and CAIx in a group of postmenopausal women compared to a group of premenopausal women [12].
Hypertension is associated with cerebral blood vessel stiffening, indirectly measured by decreased compliance of cerebral blood vessels and impaired autoregulation of cerebral blood flow [13]. Impairment of neurovascular coupling between cerebral blood flow, oxygen consumption and the brain’s electrical signaling networks may explain why stiffness of cerebral blood vessels is associated with cognitive decline [14]. Some predilections for hypertension appear to involve interactions between the genetic loci for NO synthase-3 and the beta-2 adrenergic receptor [15]. A nitrate-rich dietary treatment, such as BJ, which increases blood NO might, therefore, protect against both hypertension and cognitive decline [8]. We have previously shown that a single BJ treatment decreases myocardial oxygen demand during aerobic exercise [16–18] and that BJ decreases vascular resistance index measured in the Middle Cerebral Artery (MCA) by TCD ultrasonography [16]. TCD-measured resistance and pulsatility indices are increased by aerobic exercise. Inverse associations between carotid flow pulsatility index and brain volumes and between pulsatility index and cognitive function are thought to arise from an impedance mismatch in the cerebral microcirculation [19]. It is noteworthy that pulsatility index is positively correlated with the volume of white-matter hyperintensity, in elderly subjects associated with aging [20]. Ultrasonographic measurement of MCA blood flow velocity (MCAV) with computation of CAIx, a measure of the reflected flow signal, is shown to be modulated by changes in cerebrovascular resistance and compliance, a putative effect of dietary nitrate supplementation [20].
The present study was designed to test the hypothesis that a single BJ treatment may decrease CAIx, thereby suggesting a mechanism for improvement in cognitive functions associated with dietary nitrate supplementation.
Materials and Methods
This study used a crossover design where participants were randomly assigned to receive either a placebo control OJ or a BJ treatment. The subjects were of African-American descent and physically active but were not involved in a regular physical exercise training program, were not users of tobacco products or consumers of alcohol and were disease-free. The Institutional Human Participants Review Board of Howard University, USA had approved the study protocol.
The rationale for restricting the study to women was: 1) Women are at higher risk for poor outcome from strokes than men [21] and there is a paucity of research on healthy women subjects; 2) Plasma estrogen levels are correlated with transcranial Doppler pulsatility index [22] and no such relationship has been shown for men with respect to plasma testosterone in men; 3) Estrogen is a potent vasodilator and we predicted that, by increasing blood NO, the effects of a BJ treatment would be enhanced in women compared to men, related to the relatively higher plasma estrogen levels.
All the participants were explained of the experimental procedures and study risks and were enrolled after they gave their written informed consent. Percentage of body fat was measured by dual energy X-ray absorptiometry (GE Lunar, Madison, WI). The potential for bias was controlled by presenting this study to the participants as a procedure measuring their energy expenditure during exercise after ingesting and metabolizing two different beverage energy substrates in the form of BJ and OJ.
Subjects arrived at the laboratory on two separate days, (Jan-Dec, 2013) approximately 1-2 weeks apart in a fasting condition. Subjects were instructed to refrain from exercise for 12 h and upon arrival to the laboratory were placed in a sitting position while ingesting the 500 mL Beetroot Juice (BJ) or Orange Juice (OJ), consisting of 220 Cal according to the suppliers. The BJ treatment contained 1500 mg/L nitrate (CAJ Food Products, Inc., Fishers, IN); nitrate in the OJ is nil (Tropicana Products, Inc., Bradenton, FL). In order to digest, absorb and metabolize the beverages, they were ingested 120 minutes before the testing [23–25]. During the postprandial period, before testing, the subjects remained under supervised conditions of rest in the laboratory and did not ingest food or fluid.
Exercise Protocol and Ergometry Tests: All subjects performed a progressive exercise test of peak oxygen consumption (VO2peak on an electronically-braked leg cycle ergometer (Lode Corival, Groningen, Netherlands). The initial workload began at a level of 20 W for 3 min and was increased by equal work intensities every 3 min to volitional fatigue. Before the study, the cycle ergometer was calibrated for power outputs of 10–1000 W. During the test, Blood Pressure (BP) was determined noninvasively using the SunTech 4240 (SunTech Medical, Inc., Morrisville, NC) automated sphygmomanometric device which measures BP by gating the R-wave with the Korotkoff sounds. Heart rate (HR) was measured by an electrocardiogram with three electrodes placed at the RL, LA, and V5 anatomical sites connected to the automated BP monitor. In the exercise tests, oxygen consumption (VO2), minute ventilation (V.E), the carbon dioxide excretion rate (VCO2), and the respiratory exchange ratio (R) were measured breath-by-breath by a computerized metabolic system (Physio-Dyne Max II, Quogue, NY). The VO2 measured during the last minute of the progressive exercise test was defined as peak oxygen consumption (VO2peak). Prior to each test, the gas analysers and respiratory flow meters were calibrated with high-precision calibration gases (20.99% ± 0.01% O2 and 5.00% ± 0.01% CO2; Scott Medical Products, Plumsteadville, PA) and a 3-L calibration syringe (Hans Rudolph, Shawnee, KS), respectively. After the VO2 test, the subjects performed two separate submaximal ergometry tests under identical conditions on different days, after ingestion of the experimental supplement BJ or the control OJ. Five min after sitting rest, baseline measures of heart rate, systolic and diastolic BP, and VO2 were recorded. The subjects then completed three bouts of exercise at the constant submaximal workloads corresponding to 40% and 80% of their predetermined VO2peak values with every workload lasting 5 min. The cardiovascular and cerebrovascular responses to the three submaximal aerobic exercise workloads were measured in a stepwise fashion, with no rest intervals in between, by maintaining constant ergometer settings corresponding to 40% and 80% of each subject’s predetermined VO2peak for 5 min. Heart rate (HR), BP and VO2 were measured at min 4 and 5 of each exercise workload, with the mean values used for analysis. All the submaximal ergometry tests were performed during the luteal phase of the menstrual cycle to eliminate confounding influences of hormonal changes on BP [17,18].
Cardiac Output Measurement: Cardiac output was measured noninvasively by using the CO2 rebreathing technique. End-tidal partial pressure of CO2 (PCO2) was measured using a rapid-response infrared CO2 analyser (PHYSIODYNE Instrument Corp., Quogue, NY). End-tidal PCO2 was used to estimate arterial PCO2 [26–28]. Subjects re-breathed into a 5-L latex bag containing CO2 and O2 to permit rapid equilibration with venous PCO2. A valid equilibrium during re-breathing was measured by observing the plateau in PCO2 recording. This criterion requires a valid equilibrium during re-breathing is measured by observing a plateau in PCO2 that does not vary by more than ±1 mmHg. An automated gas mixing apparatus was used to adjust the initial gas volume and initial PCO2 in the re-breathing bag. If a trial had to be redone, end-tidal PCO2 values were allowed to return to baseline values before the procedure was repeated. Cardiac output was calculated from the indirect Fick equation, as follows:
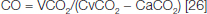
Where CO was cardiac output, VCO2 was expired carbon dioxide, CvCO2 was mixed venous CO2 content, and CaCO2 was arterial CO2 content.
Transcranial Doppler Ultrasonography: Cerebral Blood Flow Velocity Measurement was done as in our previous study [16]. The right Middle Cerebral Artery Mean Blood Velocity (MCAV) was measured with transcranial Doppler ultrasonography (Pioneer Series 4040 Transcranial Doppler Ultrasound; Nicolet Vascular, Madison, WI). Beat-by-beat TCD signals were analysed offline using the 4040 system diagnostic software. Cerebrovascular Resistance Index (CVRI) was computed as MCAV/mean arterial BP. According to the method described by Kurji et al., we used Fast Fourier transform (FFT) filtered recordings of MCAV waveforms and computed an MCAV augmentation index (CAIx) as follows [12]:
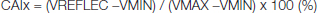
Where VREFLEC was velocity at the reflected shoulder of peak systole, VMIN was the minimum velocity and VMAX was the maximum velocity at peak systole.
End-Tidal Carbon Dioxide Tension (PCO2) Measurement. Because of the dependence of MCAV on arterial carbon dioxide, respiratory gas analysis of end-tidal PCO2 was measured on a breath-by-breath basis using an Invivo MAGNITUDE 3150 monitoring system (Invivo Research, Inc., Gainesville, FL). The monitor consists of an infrared CO2 analyser and was calibrated before each study.
Statistical Analysis
Results were expressed as mean±SEM. A two-way repeated-measures analysis of variance was performed to evaluate the difference between the supplement and control conditions; factor 1 was specified as control OJ versus BJ treatment and factor 2 as exercise level using SPSS software (SPSS Inc., Chicago, IL, USA). Pearson’s product-moment correlation analyses were performed across the blood {NO} and other study outcome measures. Data describing the study group characteristics were reported in [Table/Fig-1] as mean ± Standard Deviation (SD) and data presenting the effects of the BJ treatment were shown in [Table/Fig-2] as mean ± standard error (SEM). The significance level was set at p< 0.05.
Clinical and demographic characteristics of study subjects.
| Variable | Mean ±SD |
|---|
| Age (years) | 20.5 ± 0.6 |
| Height (cm) | 159.5 ± 5.7 |
| Body weight (kg) | 58.7 ± 6.3 |
| Systolic blood pressure (mm Hg) | 117.3 ± 7.6 |
| Diastolic blood pressure (mm Hg) | 87.2 ± 5.1 |
| Heart rate (beats/minute) | 86.3 ± 13.0 |
| VO2 peak (mL/kg/minute) | 26.3 ± 3.8 |
| Body fat (%) | 29.0 ± 5.7 |
VO2peak – peak oxygen consumption.
Effects of experimental beetroot and control orange juice treatments.
| Variable | Baseline (Rest) |
|---|
| Orange Juice | Beetroot Juice |
|---|
| Heart rate (beats/minute) | 85.7 ± 4.3 | 84.4 ± 4.3 |
| Systolic blood pressure (mm Hg) | 119.2 ± 3.1 | 112.2 ± 3.0* |
| Diastolic blood pressure (mm Hg) | 88.6 ± 2.7 | 86.0 ± 2.1 |
| MCAV (cm/second) | 89.6 ± 3.2 | 83.8 ± 5.6 |
| Cardiac output (L/minute) | 4.8 ± 0.16 | 4.8 ± 0.14 |
| PETCO2 (mm Hg) | 40.0 ± 0.6 | 39.7 ± 0.7 |
| 40% VO2peak |
| Heart rate (beats/minute) | 107.0 ± 4.1 | 106.0 ± 3.9 |
| Systolic blood pressure (mm Hg) | 129.0 ± 4.8 | 121.0 ± 5.8* |
| Diastolic blood pressure (mm Hg) | 80.4 ± 2.3 | 80.1 ± 3.8 |
| MCAV (cm/second) | 95.1 ± 2.2 | 99.1 ± 8.2 |
| Cardiac output (L/minute) | 6.3 ± 0.25 | 6.7 ± 0.26 |
| PETCO2 (mm Hg) | 39.0 ± 0.9 | 39.0 ± 0.6 |
| 80% VO2peak |
| Heart rate (beats/minute) | 155.6 ± 3.7 | 154.0 ± 3.7 |
| Systolic blood pressure (mm Hg) | 161.0 ± 6.2 | 151.0 ± 4.0* |
| Diastolic blood pressure (mm Hg) | 85.2 ± 5.2 | 83.4 ± 2.7 |
| MCAV (cm/second) | 120.7 ± 4.0 | 124.0 ± 7.0 |
| Cardiac output (L/minute) | 10.5 ± 0.5 | 10.8 ± 0.6 |
| PETCO2 (mm Hg) | 36.3 ± 1.0 | 37.0 ± 1.0 |
MCAV = middle cerebral artery blood flow velocity, PETCO2 = end-tidal partial pressure of carbon dioxide. Data in mean ± SEM. *p<0.05, Control Vs BJ.
Results
[Table/Fig-1] presents the demographic and physiologic characteristics of the study group showing that the study subjects were healthy young-adult females within a narrow range of age (20.5±0.6 y). Another characteristic of the study group was sedentary lifestyle by virtue of the relatively high percentages of body fat (29.0± 5.7%), resting heart rates (86.3±13.0 beats/min and diastolic blood pressure observed (87.2±5.1 mm Hg).
[Table/Fig-2] shows that the BJ treatment decreased systolic BP at rest and at the two levels of exercise studied, 40% VO2peak and 80% VO2peak (p<0.05). The OJ treatment had no significant effects on systolic BP and there were no significant OJ or BJ treatment-related effects on diastolic BP, HR, CO, MCAV, or PCO2.
[Table/Fig-3] presents the TCD-measured pulsatility and resistance indices (PIx, Rix) at rest and at the two levels of exercise increases in both PIx and RIx were observed during the aerobic exercise after both the OJ and BJ treatments. The BJ-induced increases in PIx and RIx were not significantly different than the OJ-induced increases.
Effects of Orange Juice and Beetroot Juice Treatments on TCD Pulsatility and Resistance Indexes.
| Variable | Mean ± SEM | p-value |
|---|
| Pulsatility Index – Orange Juice (Control) |
| Baseline (Rest) | 0.77 ± 0.04 | |
| 40% VO2peak | 0.96 ± 0.06* | <0.01 |
| 80% VO2peak | 1.09 ± .07* | <0.01 |
| Pulsatility Index – Beetroot Juice |
| Baseline (Rest) | 0.73 ± 0.03 | |
| 40% VO2peak | 0.87 ± 0.04* | <0.01 |
| 80% VO2peak | 1.11 ± 0.06* | <0.01 |
| Resistance Index – Orange Juice (Control) |
| Baseline (Rest) | 0.50 ± 0.01 | |
| 40% VO2peak | 0.57 ± 0.02* | <0.05 |
| 80% VO2peak | 0.61 ± 0.02* | <0.05 |
| Resistance Index – Beetroot Juice |
| Baseline (Rest) | 0.48 ± 0.01 | |
| 40% VO2peak | 0.54 ± 0.01* | <0.05 |
| 80% VO2peak | 0.61 ± 0.01* | <0.05 |
TCD = transcranial doppler; VO2peak = peak oxygen consumption; *Baseline Vs Treatment.
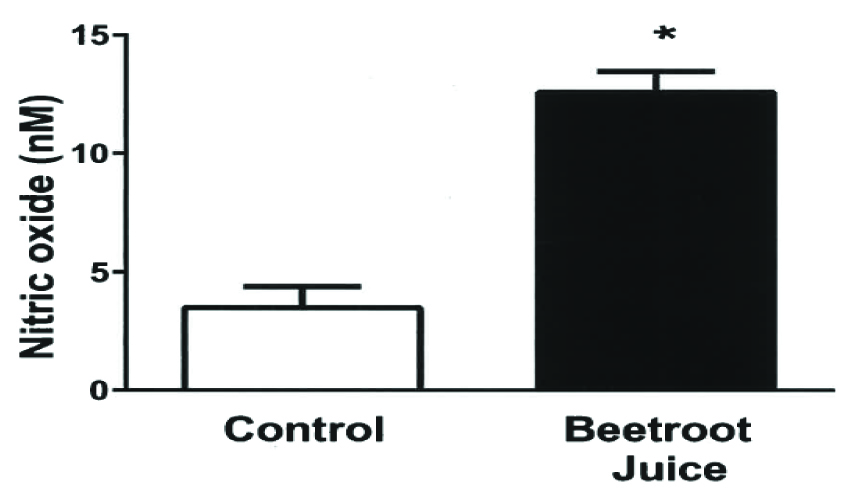
[Table/Fig-4] shows the greater blood NO concentration associated with the BJ treatment compared to the OJ control (13 ± 1 vs. 4 ± 1 nM, p<0.001).
Effects of orange and beetroot juice (OJ, BJ) treatments on blood nitric oxide concentration. Bars represent the mean ± standard error values for the blood nitric oxide (NO) level, expressed in nM. The blood NO level was measured in 10 healthy African-American female subjects at rest 2 h after ingesting isocaloric experimental beetroot and control orange juice beverages on separate days. *p<0.001, Control Vs BJ.
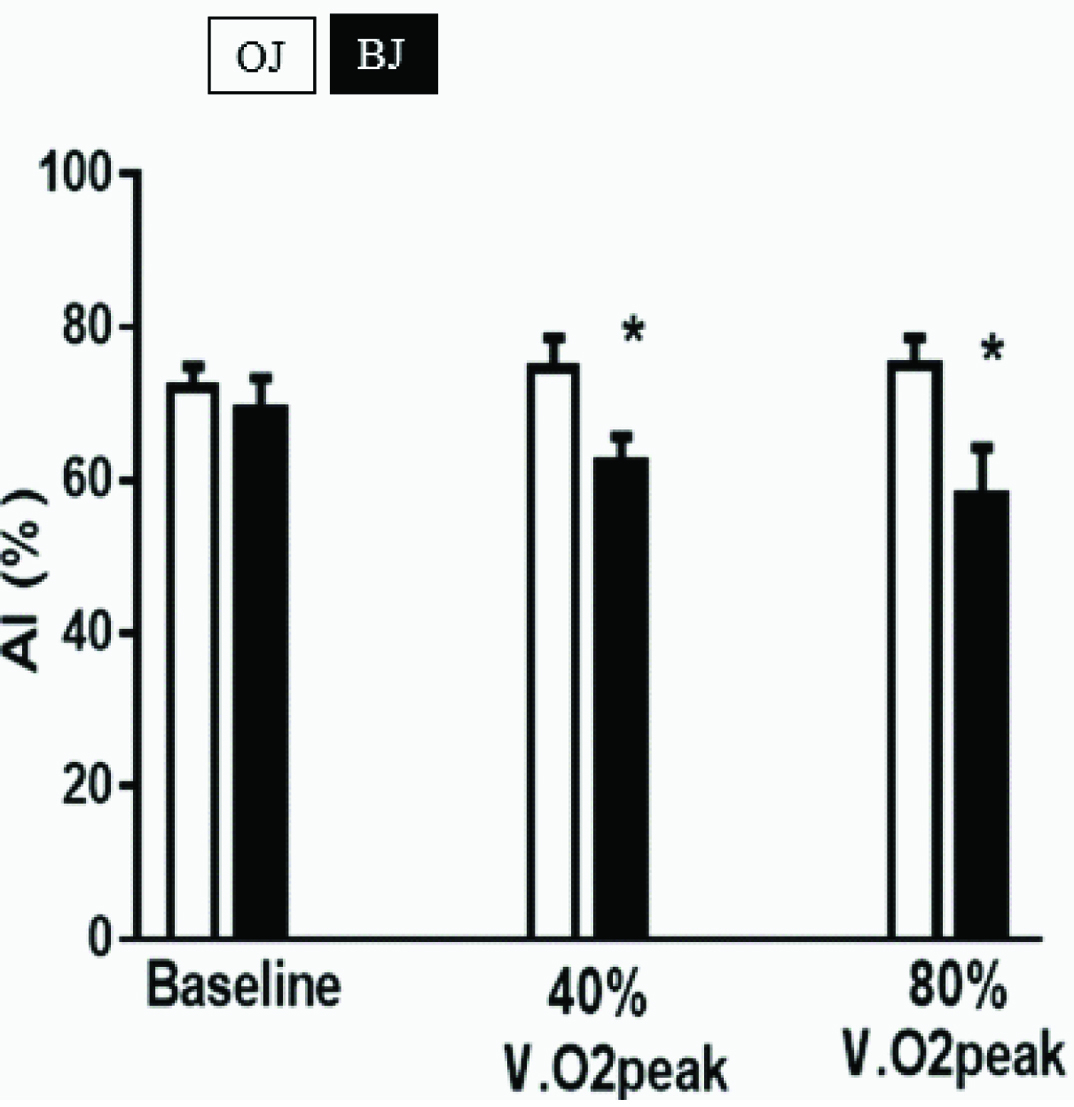
[Table/Fig-5] demonstrates that the BJ treatment decreased CAIx at the two levels of exercise studied, from 79 ± 2% to 62 ± 2% at 40% VO2peak, and from 80 ± 2% to 60 ± 3% at 80% VO2peak. There were no significant OJ or BJ treatment-related effects on CAIx at rest.
Effects of orange and beetroot juice treatments on cerebrovascular stiffness. Bars represent the mean ± standard error values for the cerebrovascular Augmentation Index (AI), expressed in percent. AI was computed from transcranial Doppler ultrasonographic measurements of middle cerebral artery blood flow velocity waveforms. The measurements were for 10 normotensive, healthy African-American females at rest (baseline) and while exercising on a cycle ergometer apparatus at 40% and 80% of their predetermined peak oxygen consumption (VO2peak), 2 h after ingesting isocaloric experimental beetroot and control orange juice beverages on separate days. *p<0.05, OJ Vs BJ.
Discussion
The main finding of this study was that CAIx decreased 20%-25% at two levels of aerobic exercise, following a dietary BJ, nitrate-rich treatment which increased the blood NO level three-fold. An isocaloric OJ treatment, which had no effect on the blood NO level, served as the control. We performed waveform analysis and computed CAIx similarly to the methods which showed greater cerebrovascular stiffness in postmenopausal, than in premenopausal, women [12]. The novelty of the present study lies in the measurement of augmentation index which suggests that middle cerebral artery stiffness is reduced, a new finding in this cohort.
The BJ treatment had no significant effects on ventilation evidenced by absence of a BJ treatment-related effect on end-tidal PCO2, a control variable for MCAV measurements [29] and an important regulator of CSF pressure. The BJ treatment-related effect of decreasing CAIx was observed only during the two levels of aerobic exercise, not during a baseline resting condition. There was no BJ treatment-related increase in body height or heart rate or decrease in total peripheral resistance that could account for the finding of decreased CAIx during exercise. However, the BJ treatment did decrease systolic BP at rest and during exercise. Increased BP associated with exercise is shown to provide a low-frequency perturbation of cerebral blood flow velocity which correlates with stiffening of the arterial blood vessels [29,30]. Thus, the BJ treatment-related decrease in systolic BP should have lessened cerebral arterial stiffening observed during exercise.
The absence of a BJ treatment-related effect on CAIx at rest is one of the more interesting aspects of this study. A plausible explanation for our inability to detect a BJ treatment-related effect on CAIx at rest is that, compared to exercise, baseline carotid pressures and flows to the MCA might not have produced enough turbulence to recruit the energy-dissipating functions of the circle of Willis, carotid siphon, dural sinuses and CSF. This interpretation implies that increased blood NO, NO bioavailability and NO-induced vasorelaxation associated with the BJ treatment likely increased the effectiveness of the aforementioned energy-dissipating functions of the circle of Willis.
The possible mechanism of the increase in blood NO following treatment with the beetroot juice may be using L-arginine as the substrate, NO production is catalyzed by three isoforms of Nitric Oxide Synthase (NOS) which includes endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) enzymes. NO is also produced from dietary sources, mainly green leafy vegetables such as spinach, arugula and beetroot, by a mechanism known as the nitrate-nitrite-NO pathway involving the sequential, nonenzymatic reduction of nitrate [31]. It is noteworthy that, despite increased MCA PIx, CAIx remain unchanged from the baseline resting to the two levels of aerobic exercise conditions following the control OJ treatment, associated with low blood NO levels. This finding bolsters our argument that the combination of high blood NO level and exercise was instrumental in enhancing the energy-dissipating functions of the circle of Willis and related structures and dampening the effects of high pulsatility during exercise, evidenced by decreased CAIx.
The results of this study raise the question as to whether the BJ-induced decreases in CAIx are indicative of a beneficial or a deleterious effect. Wave reflections exist for several reasons, one of them being to protect the cerebral microcirculation and brain parenchyma from excessive pressure pulsatility is deleterious and can cause end-organ damage. This is especially important in organs whose circulation is characterized by high-flow and low-impedance, such as the brain. As such, increased cerebrovascular pulsatility is associated with cerebral arteriosclerosis, subcortical infarcts, and lower brain volumes [19] and cognitive dysfunction [32]. Thus, by artificially decreasing wave reflections, and as such decreasing CAIx at the level of the cerebral microcirculation and brain parenchyma, we run the risk of allowing greater pressure pulsatility to penetrate the cerebral circulation, which could be deleterious. Indeed, in this study, we observed that arterial pulsatility increased with exercise even after the BJ treatment. Thus, based on this study, we cannot conclude that BJ-induced decreases in CAIx are a beneficial mechanism; as it may, in fact, prove to be deleterious – only prospective studies will be able to answer this question.
Limitations
The limitation of this pilot study is the small sample size.
Conclusion
Arterial stiffness is thought to be an important cofactor in the flow-resistive properties of blood vessels and in the co-morbidities of hypertension and cognitive decline, usually measured with subjects at rest. In view of recent findings that BJ treatments improve athletic and cognitive performances, the effects of dietary nitrate supplementation on the brain during conditions of rest and exercise appears to be worthy of further study. Dietary guidelines for the clinical management of hypertension-related cognitive decline are lacking and future studies should determine whether dietary nitrate supplementation is a safe, effective adjunct to current antihypertensive treatments for lowering BP, lessening cerebrovascular stiffness and ameliorating cognitive decline.
VO2peak – peak oxygen consumption.
MCAV = middle cerebral artery blood flow velocity, PETCO2 = end-tidal partial pressure of carbon dioxide. Data in mean ± SEM. *p<0.05, Control Vs BJ.
TCD = transcranial doppler; VO2peak = peak oxygen consumption; *Baseline Vs Treatment.