The optic nerve head is formed by the retinal nerve fibers exiting the globe. The blood flow to the optic nerve head is dependent on the perfusion pressure, which in turn is determined by the systemic blood pressure and Intra Ocular Pressure (IOP). Altered autoregulation of retinal circulation in hypertension and hypotension (due to overzealous treatment) leading to hypoperfusion and ischemia of the tissues is well known [1–3]. Similarly, nocturnal hypotension causing worsening of glaucoma with associated Retinal Nerve Fibre Layer (RNFL) loss is also well documented [4–6].
Several studies have identified the effect of varying blood pressure on the optic nerve blood flow [7–10]. LALES data and various other studies have shown that high systemic blood pressure, low systolic, diastolic and mean ocular perfusion pressure have led to increased risk of developing optic nerve damage [11,12]. In addition various studies have shown that antihypertensive agents would decrease the ocular perfusion pressure which in turn could have a potentially damaging effect on the ONH perfusion [2,3,13,14]. Study by Khawaja et al., did not find any positive association of RNFL loss and hypertension [15]. However, recently in 2015, two studies have reported RNFL loss in hypertensives [16,17]. Since, there are not many studies assessing the RNFL thickness in hypertensive patients, the current study aimed at analysis, comparison and correlation of the RNFL thickness in hypertensive and normotensive individuals. The study results may form the basis of a new evidence in interpretation of RNFL change in hypertensives similar to the revolution made by the RNFL assessment in the diagnosis of pre-perimetric glaucoma.
Materials and Methods
This cross-sectional study was conducted in a tertiary care centre from October 2012 to September 2014. Patients diagnosed to have hypertension and age matched normotensives were enrolled in the study. As there were no reports of association of RNFL thickness and hypertension during the commencement of the study, a pilot study was done to calculate the sample size.
In order to estimate the minimum sample size, a pilot study was conducted on 20 patients (10 in hypertensive group and 10 in control group). A difference of 5 μm was obtained between the two groups. In order to detect a minimum clinically relevant difference of 5 μm in RNFL at 5% level of significance and 80% power, the minimum required sample size was found to be 29:
N= (Z 1-α + Z 1-β)2σ2 / d2
Where α= level of significance, α =0.05 or 5%, Z 1-α = 1.96
1-β= Power of the test, for 1-β = 80%
Z 1-β = 0.84, σ = 9.6 (SD), d= 5 (clinically significant difference)
Therefore, a total of 60 patients, 30 patients with systemic hypertension and 30 age matched normotensives (above 45 years) were studied. The hypertensive patients and normotensive controls between 40 to 70 years of age, visiting the eye department of the tertiary care hospital between October 2012 to September 2014 were included. Those individuals who did not give the consent, those with physical or mental disability were excluded. All those patients with any ocular (like uveitis, glaucoma, etc.,) or systemic disease (other than hypertension) and all those conditions which could affect the optic nerve were excluded. History of intraocular surgery or any kind of laser therapy including refractive surgery, Refractive error >/–4.0 or >/+4.0 D and +/- 2D cylinder; visual acuity <6/9 [18], hazy ocular media (nuclear opalescence, nuclear colour and cortical changes beyond grade 3 (NO1-3,NC1-3,C1-3) Posterior subcapsular opacity as per lens opacity classification system III) and intra ocular pressure more than 20 mm Hg were excluded.
Controls were those age matched normotensive individuals who visited the Ophthalmology Outpatient Department for routine eye check-up. Ethical committee clearance was obtained prior to the study. A written, informed consent was obtained from all the participants before enrollment. After enrollment, patients were interviewed for demographic data such as age, gender and occupation. Detailed history including the duration of hypertension and the details of anti-hypertensive medications were recorded. Systemic and detailed ocular examination of both the eyes (visual acuity, intra ocular pressure, anterior and posterior segment) was performed. To minimize any recall bias related to the use of specific classes of antihypertensive medications, subjects were asked to bring all medications that they received at the examination center. Brand names of antihypertensive medications were originally recorded in study files. The medications were reclassified into generic names and corresponding classes of antihypertensive medications.
These findings were recorded on a predesigned and pretested proforma. The blood pressure of the hypertensive patients on treatment (diagnosed by the physician) was recorded. Blood Pressure (BP) was measured by random zero sphygmomanometer with the participant in the sitting position. Two consecutive measurements of systolic and diastolic BP were obtained, and the average was used in the analysis. Pulse pressure was defined as the difference between systolic and diastolic BP and mean arterial BP as: diastolic BP+ 1/3 (systolic BP−diastolic BP). Difference between (systolic, diastolic, mean arterial) Blood pressure and IOP was defined as Ocular Systolic Perfusion Pressure (OSPP), Ocular Diastolic Perfusion Pressure (ODPP), and Mean Ocular Perfusion Pressure (MOPP) respectively.
Based on the blood pressure recording during the ophthalmic evaluation, the hypertensives were grouped into three categories: Category 1 – less than130/85 mm of Hg; Category 2- 130-139/ 86-89 mm of Hg; category 3- 140-159/ 90-99 mm of Hg.
All the patients underwent dilatation with 1% Tropicamide eye drops and all OCT images of the Retinal Nerve Fiber Layer Thickness (RNFLT) were taken by a single trained professional. The RNFL images were obtained from both the eyes. The images were taken by making the patient look at an internal fixation target. The anterior and posterior layers of RNFL were measured by in-built software. Only those patients who had dilatation more than 6mm were included. Image quality was standardized on the basis of the following : signal strength >7, RPE and retinal nerve fiber layer around optic disc seen clearly in red colour and a 3.4mm circle placed with its center at the optic disc. RNFL images of both hypertensive and normotensives images were taken [Table/Fig-1,2].
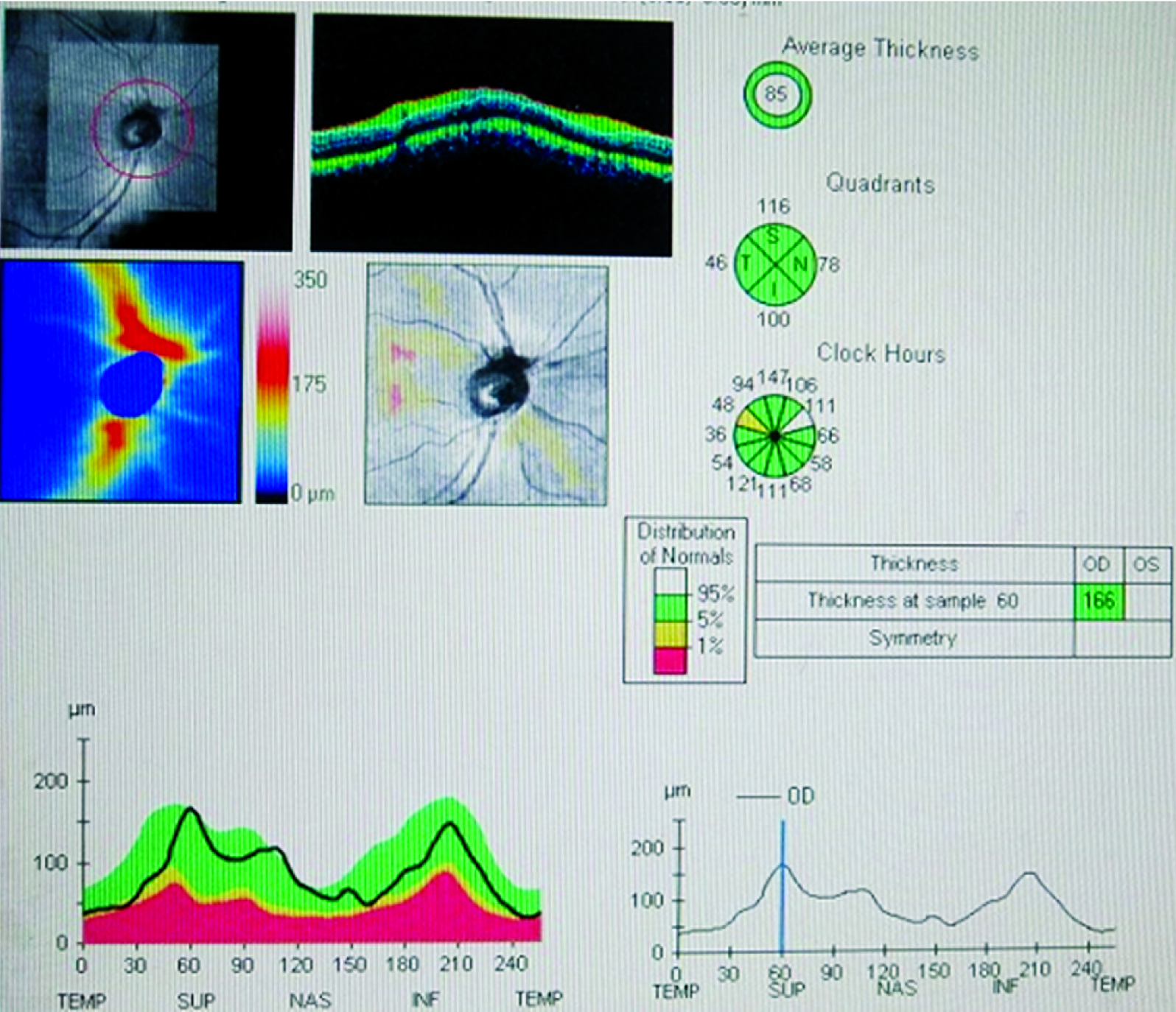
Shows the OCT image of the RNFL thickness in a Hypertensive individual. ISNT denotes the Inferior, Superior, Nasal and Temporal RNFL Thickness with the values in micrometers. The average value is also mentioned.
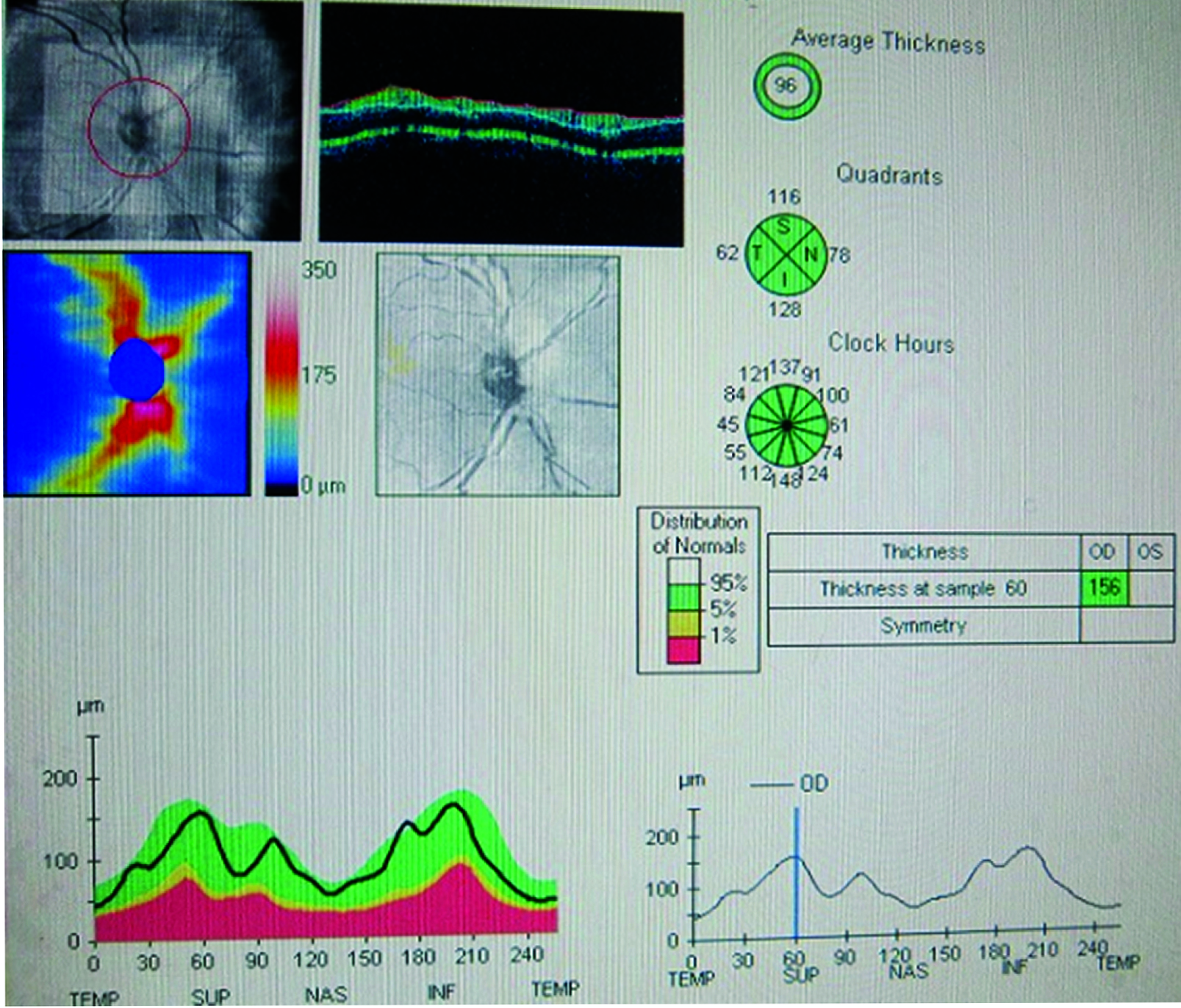
Shows the OCT image of the RNFL thickness in a Normotensive individual. ISNT denotes the Inferior, Superior, Nasal and Temporal RNFL Thickness with the values in micrometers. The average value is also mentioned.
Among the test group i.e., the hypertensives, 80% were under control and only 6 patients had comparably higher value, hence statistical analysis of that intra group variation of RNFL could not be done.
Statistical Analysis
The data was coded and compiled on Microsoft Excel spreadsheet. Categorical data was expressed in terms of rates, ratios and percentages. Continuous variables were expressed as mean ± Standard Deviation (SD). The data was analysed by paired sample t-test. A probability value (p-value) of <0.05 was considered as statistically significant.
Results
A total of 60 patients, 30 patients with systemic hypertension and 30 normotensive patients matched for age (above 45 years) were studied. The mean age among both the groups was 59.03±6.995 years [Table/Fig-3]. Overall it was observed that though the males (n=16) were more than females (n=14) in both the groups, no statistically significant difference was observed between the two groups suggesting sex distribution in the groups was comparable (p>0.05). Hypertensives on treatment were categorized based on the blood pressure control as shown in [Table/Fig-4].
Age distribution of hypertensives and normotensives.
| Age group (Years) | Hypertensives (n=30)No. (%) | Normotensives (n=30)No. % |
|---|
| 45-50 | 6 (20) | 6 (20) |
| 51-55 | 5 (16.67) | 5 (16.67) |
| 56-60 | 5 (16.67) | 5 (16.67) |
| 61-65 | 7 (23.33) | 7 (23.33) |
| 66-70 | 7 (23.33) | 7 (23.33) |
| Total | 30 (100) | 30 (100) |
Classification of hypertensives on treatment in to 3 categories depending upon their blood pressure values.
| Total number of patients (n=30)N (%) | Category |
|---|
| 24 (80) | 1 |
| 04 (13.33) | 2 |
| 02 (6.67) | 3 |
With respect to the antihypertensive treatment, it was found that the calcium channel blockers were used in 60% of the patients and beta blockers were next in line [Table/Fig-5].
Antihypertensive Treatment used by the patients is as follows.
| Total number of patients (n=30)N (%) | Treatment used |
|---|
| 18 (60) | Calcium channel blockers |
| 06 (20) | Beta blockers |
| 02 (6.67) | ACE Inhibitors |
| 01 (3.33) | Diuretics |
| 01 (3.33) | Angiotensin Receptor Blockers |
| 01 (3.33) | Calcium Channel Blocker + Beta Blocker |
| 01 (3.33) | Diuretic + Beta Blocker |
This study has shown that there was a decrease in RNFLT as age progresses, in both the groups. Maximum thickness was seen in the age group 45-50 years, while least in 66-70 years [Table/Fig-6]. There was no statistically significant difference in RNFL loss when compared with the gender in both the groups; RNFL thickness measured in the hypertensive group in males was 98.19μm and in females was 98.44μm. In normotensives, the RNFL thickness in males and females was 102.65 and 102.35μm respectively.
Co-relation between age and average RNFL thickness between the hypertensive Vs normotensive group.
| Age (years) | Average RNFLT in μm (Hypertensives) | Average RNFLT in μm (Normotensives) |
|---|
| 45-50 | 107.583 | 113.67 |
| 51-55 | 103.3 (4.3μm loss in 5 years) | 110.9 (2.8μm loss in 5 years) |
| 56-60 | 97.9 (5.4μm loss in next 5 years) | 100.75 (10.15μm loss in next 5 years)* |
| 61-65 | 95.75 (2.15μm loss in next 5 years) | 99 (1.75μm loss in next 5 years) |
| 66-70 | 89.64 (6.11μm loss in next 5 years) | 91.75 (7.25μm loss in next 5 years) |
Between 56-60 years of age in normotensive there is marked loss of nerve fiber layer.
The RNFL thickness in each quadrant according to the ISNT (inferior, superior, nasal and temporal) and the average thickness in hypertensive and normotensive individuals were compared as shown in the [Table/Fig-5]. Statistically significant RNFL loss was noted in hypertensives when compared to the normotensive individuals [Table/Fig-7].
Quadrant wise and Average peripapillary RNFL thickness in the hypertensive vs normotensive group.
| RNFLT in HypertensivesAverage ± SD (μm) | RNFLT inNormotensives (μm)Average ± SD (μm) | p-value |
|---|
| Inferior | 127.36 ± 8.89 | 132.23 ± 9.24 | 0.001 |
| Superior | 115.5 ± 6.45 | 119.7 ± 11.40 | 0.004 |
| Nasal | 81.86 ± 5.47 | 86.07 ± 10.84 | 0.002 |
| Temporal | 68.5 ± 9.41 | 72.07 ± 4.77 | 0.007 |
| Average | 98.31 ± 7.01 | 102.51 ± 8.72 | 0.001 |
p-value <0.05 was considered significant
Discussion
The systemic blood pressure and its role in the optic nerve head perfusion are well known. Various studies have shown the retinal nerve fiber loss in glaucoma, attributing it to the poor perfusion pressure due to raised intra ocular pressure. During the conceptualization of the present study, there were no data comparing the RNFL loss in hypertensives and normotensives. However, during the publication of our study results two similar studies have shown a positive correlation between hypertension and RNFL loss.
The current study had very stringent criteria to enroll the participants so as to avoid any bias regarding the results. Studies have reported increased RNFL loss with increasing age, which is consistent with our study results [15]. Our results showed that in the age group above 40 years, there was an average of 17.86 μm RNFL loss in hypertensives and an average of 21.92 μm in normotensive individuals. Surprisingly, it was seen that, there was more RNFL loss due to aging in normotensive group.
Unlike the results of the study by Schuman et al., showed that RNFLT of men were usually thinner than that of women our results did not show any significant gender-related difference in RNFL thickness [19]. Most of the patients in the present study had a history of hypertension of less than 10 years (80%) and 60% of them were on calcium channel blocker. The study by Musken et al., revealed an increased risk of disc damage in users of calcium channel blockers [13]. However, we were not able to establish such an association as statistical analysis of our data could not be performed as the different drug groups did not contain equal number of patients. In addition, the patients were not age matched for each group.
The present study showed that the patients with systemic hypertension who were on treatment had significantly thinner peripapillary RNFL thickness as compared to normotensive age matched patients. During the initiation of the study (Until 2015 June) there were no reports showing this association. Sahin OZ et al., also found that average inferior and nasal RNFL thickness was negatively associated with diastolic blood pressure [16]. However, study by Khawaja AP et al., in a large multicenter cohort study found older age, male gender, short axial length, higher BMI and pseudophakia to be associated with thinner RNFL after adjusting the possible confounders [15]. They also had studied the blood pressure and RNFL thickness and did not find a positive correlation.
Punjabi OS et al., found that systemic hypertension treated with hypotensive medications may be a risk factor for increased progression of optic nerve parameters in glaucoma suspects/ patients [2]. Gangwani RA et al., reported a higher Mean Arterial Pressure (MAP) being associated with a higher IOP and thinner global RNFL thickness [17]. A higher Diastolic Blood Pressure (DBP) was also associated with a thinner global RNFL. These two studies analysed the RNFL thickness in patients with hypertension and glaucoma.
However, in the present study patients diagnosed to have glaucoma were excluded to avoid the faulty attribution of RNFL loss to hypertension, which might be actually due to glaucomatous damage per se. Our study has shown reduced RNFL thickness in the absence of glaucoma which reiterates the fact that lower systemic blood pressure (following treatment) could be the reason for optic nerve head damage which was shown in the form of thin NRR in the Thessaloniki Eye Study [3]. This suggests that a subclinical damage may occur in hypertensive patients on treatment which may manifest itself in later years.
Although none of the patients were on night dose antihypertensive medications, assessing the diurnal fluctuations in blood pressure would be ideal, considering that nocturnal hypotensive events play an important role in the decreased perfusion of the optic nerve head. Also, as we did not assess the initial blood pressure and changes in blood pressure over the period of study, we could not correlate the RNFL thickness during the initial high blood pressure status (uncontrolled) with the current controlled blood pressure status. We consider these points as the limitations of our study.
As there are no large studies proving the evidence of RNFL loss in hypertension or hypotension (following treatment) conclusively, clinical relevance of our findings is called into question. However, antihypertensive treatment should be used with caution in hypertensive patients as this could potentially lead to a decrease in the optic nerve head perfusion.
Conclusion
As the study points to a significant RNFL loss in hypertensives as compared to normotensives, a study involving a larger and diverse clinical sample would be ideal. There is also a need to establish a correlation between the RNFL thickness and the ocular perfusion pressure.
Between 56-60 years of age in normotensive there is marked loss of nerve fiber layer.
p-value <0.05 was considered significant