Hydrosalpinx as a Rare Presentation of Synchronous Ovarian and Endometrial Carcinoma – A Case Report
Mahjabeen Khan1, Sapna Vinit Amin2, Sujatha Bagepalli Srinivas3, Roopa Padavagodu Shivananda4, Navin Patil5
1 Intern, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal, Karnataka, India.
2 Associate Professor, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal, Karnataka, India.
3 Assistant Professor, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal, Karnataka, India.
4 Associate Professor, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal, Karnataka, India.
5 Assistant Professor, Department of Pharmacology, Kasturba Medical College, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sujatha Bagepalli Srinivas, Assistant Professor, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal-576104, Karnataka, India.
E-mail: bssujata@gmail.com
Hydrosalpinx in postmenopausal woman is rare. Most commonly it is due to primary ovarian malignancy with fallopian tube involvement or primary fallopian tube carcinoma. But hydrosalpinx with no malignancy in the fallopian tube, associated with synchronous malignancy of ovary and endometrium is rare. In a postmenopausal women, hydrosalpinx is commonly due to fallopian tube malignancy or rarely pelvic inflammatory disease. We present a rare and very interesting case of 65-year-old nulliparous postmenopausal women with bilateral hydrosalpinx and pyometra who was found to have papillary serous adenocarcinoma of the ovary and endometroid adenocarcinoma of endomertrium with normal fallopian tube. One should always suspect genital malignancy with this presentation, especially in this age group.
Fallopian tube, Pelvic malignancy, Synchronous malignancy
Case Report
A 65-year-old nulliparous postmenopausal women, presented at the Gynaecology Clinic with complaints of watery foul smelling discharge per vaginum since one and a half year. She had no history of bleeding per vagina, abdominal distension or any constitutional symptoms of malignancy. Her past history was unremarkable. There was no history of previous abdominal surgeries or family history of gynaecological cancer. Clinical examination revealed normal built with nourishment and no lymphadenopathy. Temperature was 98.60F. Abdominal examination was normal with no tenderness or palpable masses. Gynaecological speculum examination showed a healthy cervix with watery foul smelling discharge through the cervical os. On per vaginal examination, uterus was atrophic and mobile without any cervical motion tenderness. There was left and posterior fornicial fullness. On per rectal examination, rectal mucosa was free.
Transvaginal ultrasound was done which revealed atrophic uterus with a collection of 2.3x2.7 cm of fluid. Right ovary measured 1.8x1.8cm, next to which there was a mass measuring 5.5x3.4cm with both solid and cystic areas. In the left adnexa there was a tubular swelling measuring 5.6x2.6 cm. Left ovary was not visualized separately. Impression was that of a pyometra with dilated tubes – hydrosalpinx or pyosalpinx. Pelvic inflammatory disease was excluded by clinical and laboratory analysis. CA-125 -6.9ug/mL, CA 19-9 -11U/ml and CEA was 1.7ng/mL, which was normal. Blood investigations showed microcytic hypochromic anaemia (Hb: 9.9g/dL) and total WBC count- 8.7 x 103/μL. C-reactive protein was 4mg/L with normal liver and renal function tests. Chest X-ray was normal and Mantoux-test was negative.
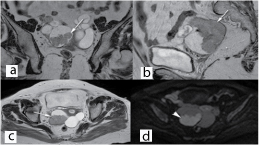
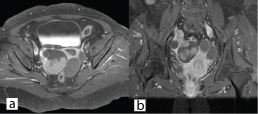
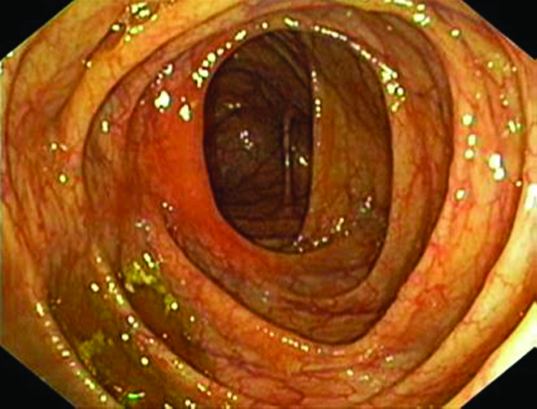
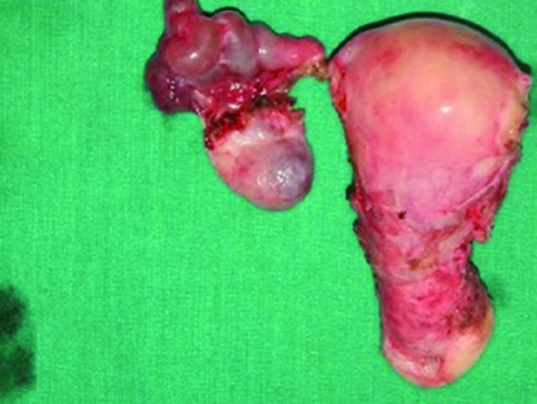
Since, the tests done were inconclusive for a particular diagnosis, plain and contrast MRI study of abdomen and pelvis was done. It showed grossly dilated bilateral fallopian tube with hypointense mass lesion arising from right ovary extending intraluminally into the right fallopian tube with minimal endometrial collection in the uterus [Table/Fig-1,2]. Possibility of right ovarian malignancy with extension into fallopian tube was thought of. MRI of abdomen revealed no evidence of GI malignancy with normal pancreatic, renal and liver morphology. Surgery consultation was sought to rule out carcinoma colon. Colonoscopy was done, which was found to be normal [Table/Fig-3]. Pap smear was negative for intraepithelial malignancy. Endometrial biopsy was not done since the endometrium was thin (3mm) on transvaginal sonography and the patient did not present with vaginal bleeding. In view of suspicious malignancy, explorative laparotomy was done. Intraoperative findings showed bilateral grossly distended fallopian tubes with enlarged right ovary and normal left ovary [Table/Fig-4a&b]. Extensive examination and sectioning of the entire fallopian tube, including fimbriae was done to ensure that small foci of malignancy in the tubes were not missed. Frozen section of right fallopian tube and ovary was done which showed papillary serous adenocarcinoma of ovary with normal fallopian tube. So total abdominal hysterectomy with bilateral salpingo-oophorectomy with infracolic omentectomy and lymph node sampling was done [Table/Fig-5].
(A-C) Coronal, Sagittal and Axial T2 weighted image reveals dilated tortuous bilateral fallopian tubes with hypointense papilleferous mass lesion arising from the right ovary and extending intraluminally into the right fallopian tube (arrow). The uterus shows minimal endometrial collection with a central hypointense foci within (dashed arrow). (D) Diffusion weighted image reveals restriction within the right tubo-ovarian mass lesion (arrowhead).
Contrast enhanced coronal and axial images reveals homogenous enhancement within the right tubo-ovarian mass lesion.
Shows normal colonoscopic finding.
(a) Shows dilated tortuous fallopian tube (white arrow) and enlarged right ovary (black arrow). (b) Shows normal left ovary.
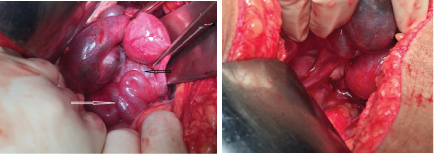
Panhysterectomy specimen showing right hydrosalpinx.
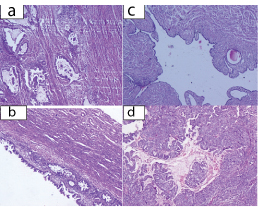
Histopathological examination was reported as papillary serous adenocarcinoma of right ovary and endometroid adenocarcinoma of uterus with insitu componentwith no malignancy in fallopian tube [Table/Fig-6]. Immunohistochemistry and immunofluorescence studies were done which showed CK-7 positive, WT -1 positive, p53 faint positive and CK-20 negative. All isolated lymph nodes, omentum, right and left paracolic – gutters and diaphragmatic peritoneum were free of malignancy. She was graded as FIGO stage IC1 of ovarian carcinoma since there was a surgical spill with no capsular ruptures involving the right ovary with endometrial carcinoma in situ. She then received 6 cycles of chemotherapy with cisplatin and paclitaxel. Patient has been coming for regular follow up. Currently she is asymptomatic and there is no evidence of disease.
(H & E; x 400): a) Endometroid Adenocarcinoma of endometrium; b) Endometrial Adenocarcinoma in situ; c) Cross section of Fallopian tube, free of tumour; d) Papillary serous adenocarcinoma of ovary.
Discussion
Synchronous malignancy of female reproductive organ is rare. If it is present, then it is commonly seen with endometrial and ovarian malignancy [1]. There is a co-existence seen in approximately 10% of all women presenting with primary ovarian malignancy and in 5% of all women with primary endometrial carcinoma [2]. The pathogenesis for synchronous endometrial and ovarian malignancy is still unclear. Some authors have hypothesized that synchronous primary tumours develop due to the response to a carcinogenic stimulus by molecular receptors which are present on the epithelia of cervix, uterus, fallopian tubes, ovaries and peritoneal surface [3]. This explains synchronous tumours of similar histology only and not of dissimilar types. Synchronous malignancy may also be due to the shared hormonal receptors (estrogen receptors) in these predisposed tissues [4]. It was found in a study that majority of the patients with synchronous malignancy had concordant endometrioid histology in endometrium and ovary and these malignancies were in early stage with low grade and had favourable prognosis [5]. Endometroid and serous combination of synchronous malignancyis rare. In our case endometroid adenocarcinoma of endometrium and papillary serous adenocarcinoma of ovary was present. The tumour is presumed to be ovarian in origin if the serous carcinoma presents as a large ovarian mass with pre-existing benign conditions like cystadenoma and endometriosis. In the absence of pre-existing conditions, it is presumed that the origin is from Mullerian epithelium which is derived from either the ovarian surface epithelium or fallopian tube. Serous Tubal Intraepithelial Carcinoma (STIC) was ruled out by doing sectioning protocols that extensively sample the tube, including the fimbriated end. It is known as the SEE-FIM (Sectioning and Extensively Examining the Fimbriated end) protocol [6]. These studies have shown that the fimbria is a major site of origin for early serous carcinomas in high-risk women. Women with synchronous primary cancers of the endometrium and ovary were young, obese, nulliparous, and premenopausal [7]. Our patient was nulliparous, elderly and postmenopausal women. The most common presenting symptoms were abnormal uterine bleeding (37%), abdominal mass (28.3%) and abdominal pain (17.4%) in a study done by Yong Kuei Lim et al., in Singapore [8]. However our patient had presented with foul smelling watery discharge with no other symptoms. Another interesting thing in our case was that CA-125 levels were normal unlike in studies where they found that majority of patients had elevated CA-125 levels [9]. Systematic surgical staging is the mainstay of the management for the patients of synchronous endometrial and ovarian cancers [10,11]. It includes: total abdominal hysterectomy with bilateral salpingo-oophorectomy, total omentectomy, appendectomy, pelvic and para-aortic lymphadenectomy and complete resection of all disease [2,10,11]. Adjuvant therapy, especially chemotherapy which is cisplatin based should be given to treat ovarian malignancy [12]. In our case total abdominal hysterectomy with bilateral salpingo-oopherectomy with lymphnode dissection and omentectomy was done. Carcinoma endometrium was insitu endometroid adenocarcinoma and ovarian carcinoma was stage IC1. She was started on adjuvant chemotherapy which was cisplatin based. A favourable prognosis was seen in patients who are of younger age with high uterine differentiation grade and early stage ovarian cancer and there was no significant difference in the survival rates of endometroid and non-endometroid type of synchronous malignancy [13]. Our patient is now disease free after 8 months of follow-up. Extensive studies are required in explaining hydrosalpinx in synchronous primary ovarian and endometrial cancer where fallopian tube is free of disease.
Conclusion
Hydrosalpinx in post-menopausal women with normal tumour markers is usually thought to be benign but one should have a high index of suspicion to rule out genital malignancy in this age group. Hydrosalpinx in this particular patient was a rare instance wherein the fallopian tube was free of carcinoma and it signifies the importance of looking for other genital malignancies.
[1]. Tong SY, Lee YS, Park JS, Bae SN, Lee JM, Clinical analysis of synchronous primary neoplasms of the female reproductive tractEur J Obstet Gynaecol Reprod Biol 2008 136:78-82. [Google Scholar]
[2]. Zaino R, Whitney C, Brady MF, Simultaneously detected endometrial and ovarian carcinomas - a prospective clinicopathologic study of 74 cases: a gynaecologic oncology group studyGynaecol Oncol 2001 83:355-62. [Google Scholar]
[3]. Dubeau L, The cell of origin of ovarian epithelial tumoursLancet Oncol 2008 9:1191-97. [Google Scholar]
[4]. Sica V, Nola E, Contieri E, Bova R, Masucci MT, Estradiol and progesterone receptors in malignant gastrointestinal tumoursCancer Res 1984 44:4670-74. [Google Scholar]
[5]. Chiang YC, Chen CA, Huang CY, Hsieh CY, Cheng WF, Synchronous primary cancers of the endometrium and ovaryInt J Gynaecol Cancer 2008 18(1):159-64. [Google Scholar]
[6]. Powell CB, Kenley E, Chen LM, Risk reducing salpingo-oophorectomy in BRCA mutation carriers: Role of serial sectioning in the detection of occult malignancyJ Clin Oncol 2005 23:127-32. [Google Scholar]
[7]. Soliman PT, Slomovitz BM, Broaddus RR, Synchronous primary cancersof the endometrium and ovary: a single institution review of 84 casesGynaecol Oncol 2004 94(2):456-62. [Google Scholar]
[8]. Lim YK, Padma R, Foo L, Chia YN, Survival outcome of women with synchronous cancers of endometrium and ovary: a 10 year retrospective cohort studyJ Gynaecol Oncol 2011 22(4):239-43. [Google Scholar]
[9]. Ma SK, Zhang HT, Sun YC, Wu LY, Synchronous primary cancers of the endometrium and ovary: review of 43 casesZhonghua Zhong Liu Za Zhi 2008 30(9):690-94. [Google Scholar]
[10]. Liu Y, Li J, Jin H, Lu Y, Lu X, Clinicopathological characteristics of patients with synchronous primary endometrial and ovarian cancers: A review of 43 casesOncol Lett 2013 5(1):267-70. [Google Scholar]
[11]. Signorelli M, Fruscio R, Lissoni AA, Pirovano C, Perego P, Synchronous early-stage endometrial and ovarian cancerInt J Gynaecol Obstet 2008 102:34-38. [Google Scholar]
[12]. Castro IM, Connell PP, Waggoner S, Rotmensch J, Mundt AJ, Synchronous ovarian and endometrial malignanciesAm J Clin Oncol 2000 23(5):521-25. [Google Scholar]
[13]. Caldarella A, Crocetti E, Taddei GL, Paci E, Co-existing endometrial and ovarian carcinomas: a retrospective clinicopathological studyPathol Res Pract 2008 204(9):643-48. [Google Scholar]