Carpal Tunnel Syndrome (CTS) is the most frequent compressive focal mono-neuropathy seen in clinical practice. Clinical symptoms comprise of numbness, tingling, burning, and/or pain associated with localized compression of the median nerve at the wrist, resulting in mechanical compression and/or local ischemia [1–7]. Bedside clinical diagnosis can be made using provocative stress tests such as Phalen’s test, Tinel’s test, hand elevation test, pressure provocation test, tethered median nerve stress test, tourniquet test and others [8,9].
Along with the subjective clinical manifestations, Nerve Conduction Studies (NCS) serve as objective supplementary modalities in the diagnosis and assessment of therapeutic effects in cases of CTS [10]. NCS are considered as gold standard for diagnosis of CTS and are being increasingly used for severity assessment with high degree of sensitivity and specificity [11–14].
Severity assessment of CTS is a crucial step for defining prognosis and therapeutic measures. Numerous studies have been conducted on the diagnostic findings on NCS and although there have been various reports on CTS grade assessment, no specific method has been established [10–16].
The aim of this study was to establish correlations between standard median nerve NCS parameters with clinical severity grading in patients with CTS.
Materials and Methods
This prospective case control study, was conducted in the Neurology Outpatient Department, Bangalore Medical College and Research Institute, Bangalore with a suspected diagnosis of CTS over a period of ten months from August 2012 to June 2013. Written informed consent was obtained from all patients and healthy control subjects and the study was approved by the Institutional Ethics Committee.
Consecutive patients aged ≥ 18 years with CTS based on clinical features were included in the study and subjected for further electro-diagnostic evaluation. Patients were screened for symptoms suggestive of CTS and clinical provocative tests of CTS were done. Phalen’s test and Tinel’s test were performed in all patients. The criteria for the clinical diagnosis of CTS are elaborated in [Table/Fig-1] [4–7].
The criteria for the clinical diagnosis of CTS.
| Clinical Symptoms |
|---|
| 1 | Numbness of hand and first three fingers. |
| 2 | Severe pain that radiates from the wrist to the shoulder. |
| 3 | Tingling in their hand and fingers. |
| 4 | Falling of objects from the hand because of lack of sensation. |
| 5 | Frequent awakenings during the night due to sensory symptoms. |
| Clinical Signs |
| 1 | Tinel’s sign |
| 2 | Atrophy (wasting) of the thenar eminence |
| 3 | Phalen’s test (wrist flexion test) |
A clinical diagnosis of CTS was made when at least one of the above mentioned clinical symptom along with one or more of the clinical signs were present. The patients were divided into mild, moderate and severe grades depending on the severity of clinical symptoms as per Mackinnson’s classification as depicted in [Table/Fig-2] [4].
The Clinical severity grading of CTS.
| Clinical severity grades |
|---|
| Mild | Presence of only sensory symptoms(pain, paresthesias and/or numbness). |
| Moderate | Presence of motor symptoms (weakness). |
| Severe | Presence of muscle wasting. |
Patients with clinical evidence of CTS in addition to polyneuropathy (distal sensory loss, depressed ankle jerks, reduced sural amplitude) were excluded. Patients with a history, physical examination or investigations suggestive of cervical radiculopathy (C6, C7, C8), brachial plexopathy, proximal median neuropathy, motor neuron disease, previous hand surgeries, spondylotic myelopathy, syringomyelia, stroke, multiple sclerosis and polyneuropathy even if fulfilling the clinical criteria of CTS were excluded.
All patients were investigated with complete hemogram including erythrocyte sedimentation rate, blood glucose, renal and liver function tests, lipid profile, rheumatoid arthritis factor, C-reactive protein.
The control subjects consisted of age and sex matched healthy hospital staff and volunteers who were screened with history and examination to eliminate individuals with CTS. Pregnant and postpartum women, obese individuals, manual labourers, those pursuing certain occupations known to predispose to CTS (recurrent twisting turning of hands while working, work with vibrating tools etc), those with history of hypothyroidism, rheumatoid arthritis, diabetes mellitus were not included in this study as control subjects even though they were asymptomatic, as they could be having subclinical CTS and could thereby influence NCS results.
Nerve Conduction Studies
The electrophysiological studies were performed on bilateral upper limbs in all patients and healthy control subjects utilizing an electromyography machine (RMS & EB neuro) in a shielded room, ensuring that the patient’s skin temperature was maintained at 320C atleast. Nerve Conduction Studies (NCS) were performed according to the American Association of Electro-diagnostic Medicine criteria [10–13]. The median nerve Compound Motor Action Potentials (CMAP) were recorded from Abductor Pollicis Brevis (APB) and stimulating 3 centimeters proximal to the distal crease of the wrist was followed in all patients and healthy control subjects. Median Sensory Nerve Action Potentials (SNAP) were recorded by orthodromic stimulation of 2nd digit and recording from median nerve at wrist (3 centimeters proximal to the distal crease) and ulnar nerve SNAPs were examined by stimulating 5th digit and recoding from wrist (3 centimeters proximal to wrist crease). Inching technique (motor-maximum delay in distal segment and maximum latency change) which was done by stimulation of median nerve at multiple sites across the wrist helped in localizing the site of maximum delay within the distal segment of the nerve [2]. The normative values of various NCS parameters used in this study are depicted in [Table/Fig-3] [2].
The normative values of various NCS parameters used in this study.
| NCS Parameter | NormativeValue |
|---|
| Distal latency of median nerve compound motor action potential (CMAP) | 4.4 milliseconds |
| Difference between the distal latencies of median and ulnar nerve CMAPs | ≤ 1.1 milliseconds |
| Difference between the distal latencies of median and ulnar nerve sensory nerve action potential (SNAP) | ≤ 0.2 milliseconds |
| Median CMAP amplitude | > 4.0 millivolts |
| Median CMAP conduction velocity | > 49 meters/sec |
| Median SNAP distal latency | < 3.5 milliseconds |
| Median SNAP amplitude | > 20 microvolts |
| Median SNAP conduction velocity | > 50 meters/sec |
| F-wave latency of the median nerve | < 31 milliseconds |
Statistical Analysis
The statistical analysis was carried out by the SPSS program, windows version 15.0. For qualitative variables (like gender, laterality and clinical symptoms and signs) frequencies and percentages were calculated. For quantitative variables (like age and NCS parameters) mean and standard deviation were calculated. The proportions were compared using Chi square test and fisher exact test wherever applicable. Means were compared using student ‘t’-test or ANOVA. Data were presented in tables and figures. A p-value of less than 0.05 was considered statistically significant.
Results
Over a 10 months period, 50 patients with symptoms consistent with CTS and 50 age and sex matched healthy control subjects were examined. A total of 30 patients had unilateral CTS (right upper limb in 19 and left upper limb in 11) and 20 patients had bilateral CTS thus amounting to a total of 70 upper limbs with symptoms consistent with CTS and confirmatory NCS findings in our study.
Clinical Parameters
The baseline demographic and clinical characteristics of the two groups are depicted in [Table/Fig-4]. Among the patients, females were 39 (78%), and males were 11 (22%). Female to male ratio was 3.54 to 1. Age ranged from 25 to 81 years. The mean age at presentation was 49.68±11.7 years.
Clinico-demographic details of the two groups.
| Parameters | Control subjects(n=50) | Patients(n=50) |
|---|
| Mean age (years) | 48.84±12.1 | 49.68 ± 11.7 |
| Age range (years) | 27 – 84 | 25 – 81 |
| Mean duration of symptoms (weeks) | - | 52.68 ± 99.81 |
| Gender | Male – 11Female – 39 | Male – 11Female - 39 |
| Side of involvement | - | Left -11; Right-19; Bilateral-20 |
| Severity Grade | - | Mild-16 (32%)Moderate-25 (50%)Severe-9 (18%) |
| Symptoms -Numbness | - | 46 (92%) |
| Tingling | - | 48 (98%) |
| Tinel’s sign | - | 36 (72%) |
| Phalen’s test | - | 44 (88%) |
Tingling paresthesias of hand and first three fingers were the most frequent symptoms 48 (98%); of these nocturnal only paraesthesias were reported by 16 patients (33.4%) and both nocturnal/diurnal paraesthesias by 30 patients (62.5%). It was more noticed in bilateral and right sided CTS patients. Numbness of hand and first three fingers was present in 46 (92%). 25 (50 %) patients had APB weakness and 9 (18%) had APB muscle wasting. Tinel’s and Phalen’s sign were positive in 36 (72%) and 44 (88%) patients respectively. The mean duration of symptoms at presentation was 52.68±99.81 weeks.
The most common risk factors noted after history taking and/or as per blood investigations were hypothyroidism (12%), rheumatoid arthritis (12%), diabetes mellitus (10%), hyperlipidemia (6%), work related (6%) and postpartum state (4%).
Based on clinical assessment, the study patients were divided into 03 groups with mild CTS, moderate CTS and severe CTS respectively as per Mackinnson’s classification as elaborated in the materials and methods section. Overall, 16 patients (32%) had mild CTS, 25 (50%) had moderate CTS and 9 (18%) had severe CTS clinically. Severity was more among bilateral CTS patients when compared with unilateral CTS patients. Among unilateral CTS patients, right sided CTS patients had a severe grade as compared with the left sided ones. However the p-value was not significant on both sides.
Nerve Conduction Parameters – Motor
Patients versus Controls
The motor electrodiagnostic parameters among the patients and healthy control subjects in bilateral hands and the correlation of the same with the clinical severity grades of the patients are depicted in [Table/Fig-5].
Electro diagnostic parameters – Motor.
| Electrodiagnostic Parameters | Number of patients with Right CTS | Number of patients with Left CTS | Controls - Right hands | Controls -Left hands | p-value |
|---|
| Median nerve Mean CMAP distal latency (measured in milliseconds) | 4.6±1.5 | 4.5±1.4 | 3.2±0.4 | 3.1±0.5 | Right - 0.03Left – 0.02 |
| Median nerve Compound Muscle Action Potential (CMAP) Distal Motor Latency >4.4 (measured in milliseconds) | 39 (19 right sided only + 20 with bilateral) | 31 (11 left sided only + 20 with bilateral) | - | - | <0.001 on both sides |
| Correlation of Median nerve CMAP Distal Motor Latency >4.4 milliseconds with clinical grading | Mild-16Moderate-11Severe-12 | Mild-12Moderate-10Severe-9 | - | - | Right - 0.06Left - 0.12 |
| Median nerve Mean CMAP Amplitudes (measured in millivolts) | 10.19±0.77 | 10.09±0.98 | 17.34±1.21 | 15.85±1.23 | <0.001 on both sides |
| Comparison ofCMAP Amplitudes(measured in millivolts)with clinical grading | Mild-11.50Moderate-10.76Severe-6.27 | Mild-12.00Moderate-10.14Severe-6.15 | | | Right - 0.075Left - 0.16 |
| Mean CMAP distal latency difference between median & ulnar nerves(measured in milliseconds) | 2.34±0.01 | 2.73±0.03 | 0.66±0.04 | 0.80±0.06 | <0.001 on both sides |
| Comparison of CMAP distal latency difference (>1.10 milliseconds) between median & ulnar nerves with clinical grading | Mild-12Moderate-16Severe-11 | Mild-9Moderate-12Severe-10 | | | Right - 0.77Left - 0.37 |
| Mean median nerve CMAP conduction velocity (measured in meters/second) | 46.03±1.83 | 50.61±1.14 | 55.92±2.09 | 54.28±2.11 | Right-0.001Left-0.08 |
| Correlation of Mean median nerve CMAP conduction velocity (measured in meters/ second) with clinical grading | Mild-51.31Moderate-50.68Severe-43.16 | Mild-52.01Moderate-50.52Severe-48.35 | | | Right - 0.13Left - 0.14 |
| Mean median nerve F wave Latency(measured in milliseconds) | 25.37±1.21 | 25.29±1.24 | 31.20±1.98 | 29.64±1.83 | 0.001 on both sides |
Right sided median nerve CMAP distal latency was prolonged (> 4.4 milliseconds) among 39 patients (including 19 right CTS and 20 bilateral CTS) and left sided sided median nerve CMAP distal latency was prolonged (> 4.4 milliseconds) among 31 patients (including 11 left CTS and 20 bilateral CTS). The p-value was significant in comparison with control subjects on both sides.
It is noteworthy that the latency difference between median and ulnar nerves was more prolonged in right sided CTS patients than left sided CTS patients.
In the inching technique, maximum latency jump was seen in 1centimeter below the wrist crease as seen in 48% patients and 2 centimeters below the wrist crease seen in 26%.
Clinical Severity Grades versus Motor Nerve Conduction Parameters
On comparison with clinical grading, though there was a graded increase in median nerve CMAP distal latencies and decrease in amplitudes, the correlation of either of them was not significant on both sides [Table/Fig-5]. The mean CMAP distal latency difference between median and ulnar nerves was also not significantly correlated with the clinical severity grades. However, with regards to the decrease in mean median CMAP conduction velocities, only the severe grade group showed more decrease on the right side as compared to the left sided CTS patients though the correlation was not statistically significant (p –0.13). In inching technique, maximum latency jump was seen in 52% of moderate group in 1centimeter below the wrist crease and 28% of moderate group in 2 centimeters below the wrist crease.
Nerve Conduction Parameters – Sensory
The sensory electrodiagnostic parameters among the patients and healthy control subjects in bilateral hands and the correlation of the same with the clinical severity grades of the patients are depicted in [Table/Fig-6].
Electrodiagnostic Parameters – Sensory.
| Electrodiagnostic Parameters | Number of patients with Right CTS | Number of patients with Left CTS | Controls - Right hands | Controls -Left hands | p-value |
|---|
| Mean median sensory nerve action potential (SNAP) distal latency (measured in milliseconds) | 2.43±0.09 | 2.18±0.05 | 2.75±0.04 | 2.91±0.08 | Right-0.008Left-0.027 |
| Comparison of Median SNAP distal latency (measured in milliseconds) with clinical grading | Mild- 1.32Moderate-2.61Severe-2.77 | Mild- 1.12Moderate-2.08Severe-2.96 | | | Right - 0.035Left - 0.007 |
| Mean SNAP latency difference (measured in milliseconds) between median and ulnar nerves (normal cut-off – 0.2 milliseconds) | 0.78±0.01 | 0.54±0.01 | 0.19±0.05 | 0.18±0.02 | 0.001 on both sides |
| Number of hands with CTS having mean SNAP latency difference between median and ulnar nerves > 0.2 milliseconds | 33 hands (16 with right CTS and 17 with bilateral CTS) | 29 hands (10 with left CTS and 19 with bilateral CTS) | | | Right -0.038Left – 0.021 |
| Comparison of SNAP distal latency difference between median and ulnar nerves (> 0.2 milliseconds) with clinical grading | Mild-11Moderate-19Severe-3 | Mild-13Moderate-14Severe-2 | | | Right - 0.066Left - 0.016 |
| Mean median SNAP amplitude (measured in millivolts) | 8.55±0.18 | 8.81±0.21 | 17.03±1.1 | 17.33±1.4 | <0.001 on both sides |
| Comparison of Mean median SNAP amplitude (measured in millivolts) | Mild-8.98Moderate-9.40Severe-6.88 | Mild-10.44Moderate-10.16Severe-1.04 | | | Right - 0.80Left - 0.21 |
| Mean median SNAP conduction velocity (measured in meters per second) | 34.22±1.11 | 32.09±1.07 | 52.09±2.21 | 51.21±2.3 | <0.001 on both sides |
| Comparison of Mean median SNAP conduction velocity with clinical grading | Mild-53.31Moderate-50.68Severe-43.16 | Mild-52.01Moderate-50.52Severe-48.35 | | | Right - 0.11Left - 0.56 |
Patients versus Controls
The mean SNAP distal latency difference between median and ulnar nerves (measured in milliseconds) among the patients and healthy control subjects were 0.78±0.01 and 0.19±0.15 respectively on the right side and 0.54±0.01 and 0.18±0.02 respectively on the left side (p - 0.001 on both sides). The mean median nerve SNAP conduction velocities (measured in meters/ second) among the patients and healthy control subjects were 34.22±1.11 and 52.09±2.21 respectively on the right side and 32.09±1.07 and 51.21±2.3 respectively on the left side (p < 0.001 on both sides).
Clinical Severity Grades versus Sensory Nerve Conduction Parameters
11 right sided and 14 left sided CTS patients had absent median SNAPs. A graded increase in SNAP latency was noted from mild to severe clinical grade. There was a prolongation of distal sensory latency in patients as compared with control subjects. 62% patients showed prolonged SNAP latency difference between median and ulnar nerves. Mean median nerve SNAP amplitudes were grossly reduced in left sided CTS patients with a severe clinical grade. Right sided CTS patients did not show decrease in mean amplitude with respect to the clinical grading.
Discussion
It has long been a subject of debate whether clinical manifestations of CTS correlate well with neurophysiologic findings. Clinical CTS can be confirmed using electro diagnostic techniques that document abnormalities of the median nerve fibers within the carpal tunnel [1,6,7,14].
Primary considerations include advancing age and female gender as risks of CTS [6,8]. We included 50 patients with ages ranging from 27 to 84 years with a mean age at presentation being 48.84±12.1 years. There were more patients in the higher age groups. The disability duration also reportedly increased with age, with a peak incidence between 45 and 54 years of age. Prior studies have shown significant positive correlation between age and the time of evolution of the CTS in relation to the neuro-physiological scale. Elderly patients have severe nerve compression with relatively fewer symptoms. It is suggested that there is reduced pain sensitivity with age, which is possibly due to a reduction of the nerve membrane excitability as an age-related factor [6–9].
In our study the majority of the patients were females (78%), with a female to male ratio of 3.5 to 1. Female gender is an independent risk factor for CTS and fluid retention during pregnancy or menopause is frequently associated with development of the disease. The increased incidence in women may be partly due to hormonal factors, but in general, it is believed to be related to a propensity to and higher frequency of musculoskeletal problems among women [2,3,7–9].
The usual predisposing factors in the present study were hypothyroidism (12%), rheumatoid arthritis (12%), diabetes mellitus (10 %), hyperlipidemia (6%), recurrent twisting turning of hands while working (4%), work with vibrating tools (2%) and postpartum state (4%). Studies have shown that intense repetitive motion, vibration and extreme postures of the hand and wrist during job performance may contribute to the development of carpal tunnel syndrome. These temporarily increase pressure in the carpal tunnel, which threatens the viability of the median nerve and affects normal hand function [8]. In order to eliminate the bias of subclinical CTS, asymptomatic individuals with any of these co-morbidities were not enrolled as controls in our study.
Upton and McComas used the double crush hypothesis to explain why patients with Carpal Tunnel Syndrome (CTS) sometimes feel pain in the forearm, elbow, upper arm, shoulder, chest and upper back. They also used it to explain failed attempts at surgical repairs when neither surgery nor CTS diagnosis appeared faulty. They claimed that most patients with CTS not only have compressive lesions at the wrist, but also show evidence of damage to cervical nerve roots. Hypothetically, two lesions with little or no independent clinical ramifications, when combined, lead to appearance or magnification of symptoms. By definition, a first lesion must render axons more susceptible to effects of a second, leading to more than just the combined, independent effects of two lesions [17]. Our exclusion criteria were very stringent as detailed in the materials and methods section. None of our patients had neck pain or proximal upper limb paresthesias and hence no question of double crush hypothesis in our patients.
Bilateral CTS was found in 60% and dominant hand involvement in 78% patients in our study. Bilateral carpal tunnel syndrome is a frequent finding. In this study among the unilateral cases, right side was more involved than left side, probably because cumulative and repetitive motion injuries are more common on the right side [6,8]. The dominant hand is usually affected first and produces the most severe pain. Moreover, Individuals with bilateral disease have a greater incidence of familial disease than those with either unilateral disease or no carpal tunnel syndrome.
Traditional bedside tests known as provocative tests can be easily conducted by the physician on the patient to determine the possibility of CTS. One such test is a wrist flexion test known as Phalen’s test (sensitivity = 57%-91%; specificity = 33%-86%) [5–9] that involves the patient placing their elbows on a flat surface, maintaining their forearms vertically and allowing their wrists to fall into flexion for up to one minute. Another well known test is Tinel’s sign (sensitivity= 23%-60%; specificity = 64%-87%) [5–9], whereas the physicians taps along the patient’s median nerve near the carpal tunnel. For both of the above noted tests, if paresthesia develops or increases in the median nerve distribution within one minute or less, then the test is deemed to have a positive result and CTS in the patient may be suspected. Tinel’s test was present in 72 % and Phalen’s sign in 88% patients in our study. Phalen’s test is more sensitive than Tinel’s sign as has been previously documented by Durkan et al., [9].
It is important to note that, although provocative tests and physical examination are simple and low cost methods to test for reproduction of the patient’s symptoms and to determine if CTS should be suspected, provocative tests have scarce or no diagnostic value and physical examination has inadequate predictive value if the likelihood of CTS is low. There have been no trends identified between testing positive for various provocative tests and the severity of CTS and therefore proper diagnostic conclusions based on these tests cannot be made [10–14].
Severity assessment of CTS is a crucial step for defining prognosis and therapeutic measures. Although, different authors have proposed numerous classifications on CTS grade assessment and demonstrated nice correlation between the electrophysiologic staging and the severity of clinical symptoms, no specific method has been established [5–7]. Using the Mackinnson’s clinical grading system [4], we assigned patients with clinical symptoms and signs into one of mild, moderate or severe grades, with a view of correlating the neurophysiologic differences between patients with different clinical severity grades.
NCS tend to become abnormal after significant compression leads to ischemic demyelination of the median nerve. This occurs first in the fast conducting fibers which travel deep to the flexor retinaculum [1–3]. Thus, routine NCS measuring superficial sensory branch of median nerve may fail to pick up the pathology. Thus, as per the AAEM guidelines, orthodromic mixed nerve studies and comparative studies of median sensory latency to the ulnar, or median (segments outside the carpal tunnel) sensory latencies allows the greatest accuracy for confirming the clinical diagnosis [10–14]. These techniques increase the sensitivity and specificity of diagnosing CTS. In the present study, this fact is re-emphasized with the evidence 62% patients showed prolonged SNAP latency difference between median and ulnar nerves and a graded increase in SNAP latency difference between median and ulnar nerves was noted from mild to severe clinical grade.
Prior studies have compared sensory nerve responses with the absolute median nerve motor latencies and found that the abnormalities of the former is more effective and consistent than the latter in documenting CTS [11]. This is attributed to the fact that sensory fibers have a larger proportion of large myelinated fibers, which have a higher energy requirement, and thus are more susceptible to ischemic damage [3–9].
Among the median nerve motor conductions, mean distal motor latencies of patients were more prolonged than control subjects. Prolongation of the distal motor latency did not correlate with the clinical grading as has been reported in prior studies [8,9]. Median nerve motor amplitudes were decreased in patients (more in severe grade) when compared with the control subjects indicating axon loss.
In this study, there was a trend towards greater delays in forearm CMAP and SNAP conduction velocities with increasing severity of clinical grade, but there were no significant differences seen between the three severity groups on both sides [Table/Fig-5]. This finding may reflect the fact that when CTS was pathologically advanced, in many cases neither the CMAP nor the SNAP conduction velocities could be detected. Furthermore, Mackinnon’s classification criteria are based not only on subjective symptoms, but also on the presence or absence of muscular atrophy and sensory impairment, thus making it difficult to accurately assign clinical grades [4].
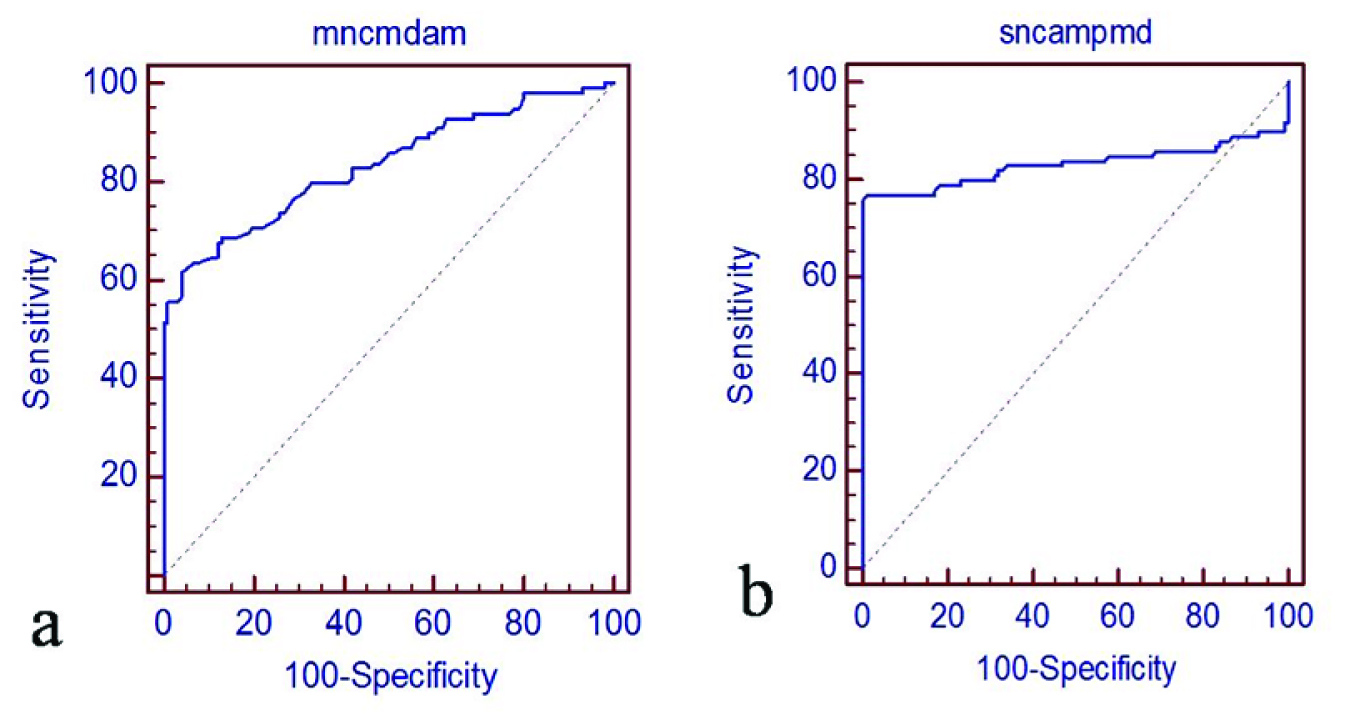
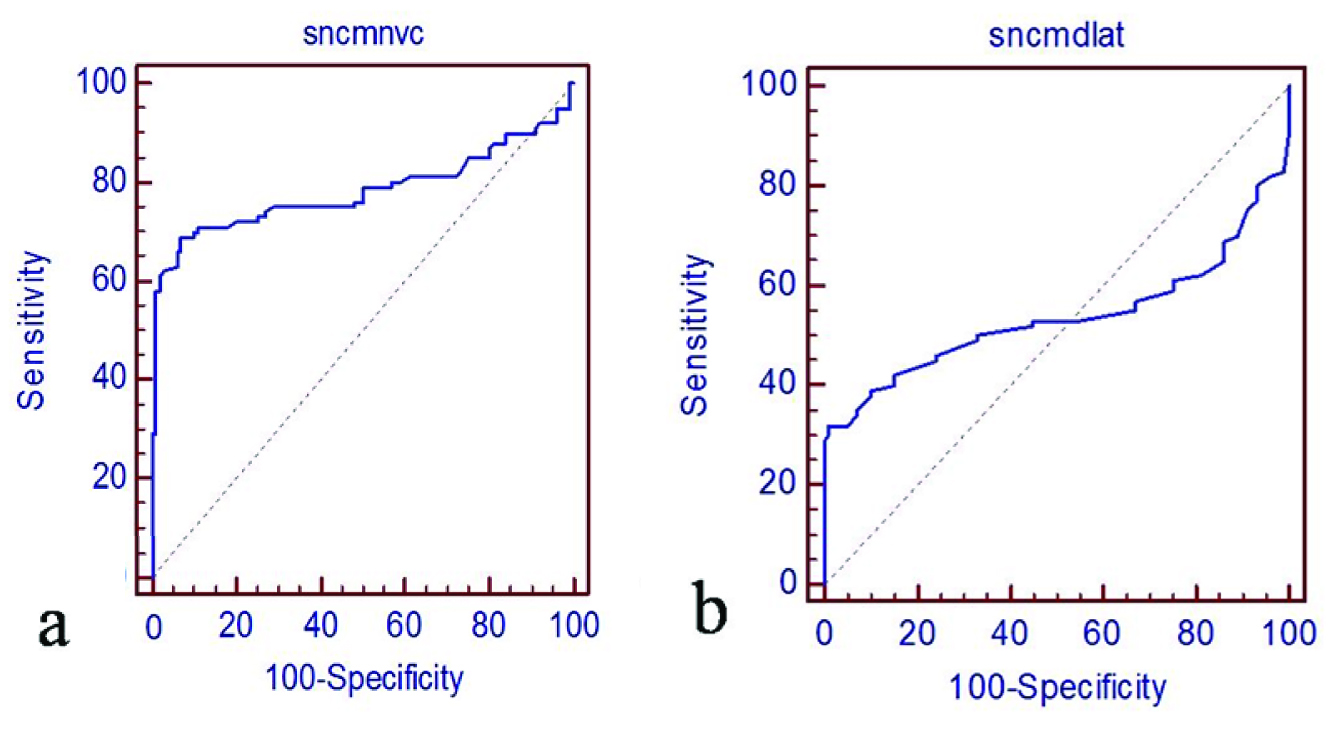
Although the amplitudes of SNAPs and CMAPs reflect the functional state of axons, the utility of SNAP/CMAP amplitude for assessing the clinical grading of CTS has not been investigated in previous studies. The primary reason for this is the technical difficulty to obtain stable waveforms suitable for assessment. SNAP/CMAP amplitudes provide a better, more reliable indicator of the clinical grading of CTS [10–13]. This is the most important parameter in NCS for CTS with respect to therapy selection. In this study, there were weak positive correlations between CMAP amplitudes and SNAP amplitudes (R2=0.83 and 0.83) [Table/Fig-7] and between SNAP distal latency and CMAP conduction velocity (R2=0.53 and 0.63) [Table/Fig-8] respectively.
Relationship between nerve motor amplitude and sensory nerve amplitudes. Weak positive correlations between (a) motor nerve amplitude (R2 = 0.83) and (b) sensory nerve amplitude (R2 = 0.83) are revealed.
Relationship between nerve motor conduction velocity (R2 = 0.63) and sensory nerve distal latency (R = 0.53).
As with the sensory nerve action potential, there was a tendency for a greater reduction of amplitude of the compound motor action potential to be associated with a more severe clinical grade. The CMAP distal latency did not correlate with clinical grading. Motor nerve latency difference between median and ulnar nerves is a sensitive indicator of CTS but not for clinical grading. The motor inching technique which is a very sensitive and specific showed maximum latency jump is seen in 1 centimeter below and 2 centimeters below the wrist crease, but it may be painful and time consuming.
In comparison with clinical grading, the mild group had more prolonged sensory latencies (left > right) but in 25 hands (right-11; left-14) there were absent sensory waves.
The comparison of median sensory latency to the ulnar, or median (segments outside the carpal tunnel) sensory latencies allows the greatest accuracy for confirming the clinical diagnosis and helps to control for other confounding variables such as temperature, age, height, and other patient-specific variability. Known as the ring diff, this is usually an antidromic technique to stimulate the median and ulnar nerve at the wrist and record 14 centimeters from the ring finger using ring electrodes. The ring finger has median and ulnar innervation, and thus comparison of these latencies can be an efficient method of establishing a relative slowing of the median nerve compared with the ulnar across the wrist. The median nerve fibers to the ring finger may be more subject to compression due to the position of ring finger fibers in the outer margin of the median nerve beneath the transverse carpal ligament [1–3,10–14].
Sensory nerve action potential amplitude has not been reported as reliable in the diagnosis of CTS as it has considerable variability [14–16]. Because the SNAP amplitudes are small, the median response may be absent in more severe cases of CTS [15,16].
Limitation
The limitations of our study were: a) small sample size which may be due to the stringent exclusion criteria; b) probable influence of various forms of treatment being received by the patients including physiotherapy, drugs for neuropathic pain, wrist splints and therapy for underlying co-morbid factors. However, none of our patients had received local corticosteroid injections or underwent surgery for CTS prior to enrollment and NCS.
Conclusion
In this study, female predilection, higher age, sensory symptoms (paraesthesias and numbness) and dominant hand involvement were more commonly associated with CTS. There was a graded deterioration of electrophysiological parameters along with the clinical severity grades, thus reiterating the fact that NCS provide additional, independent objective evidence in the diagnosis and severity assessment of CTS. The sensory conductions were more sensitive than motor conductions in assessing severity of CTS and the sensory latency difference between median and ulnar nerves and sensory nerve conduction velocities were the most sensitive and specific for diagnosing CTS. There was a weak positive correlation of motor nerve amplitude and sensory nerve amplitude with the severity of CTS, which probably indicates the functional state of axons.