Diabetes Mellitus (DM) is a chronic metabolic disease occurring as a result of qualitative and / or quantitative defect in insulin function, as a result of which blood glucose levels rise. This in turn produces polyuria, polydipsia and polyphagia [1,2]. In 2014, global prevalence of diabetes was estimated to be 9% among adults aged 18+ years and 1.5 million worldwide deaths being directly attributed to DM, making it 7th leading cause of death worldwide [3,4]. India referred to as diabetic capital of world [5] currently faces an uncertain future in relation to potential burden that diabetes may inflict upon the country. It is predicted that by 2030, DM may affect up to 79.4 million individuals in India while the diabetes population of whole world is expected to almost double from 171 million to 366 million [6]. Lifestyle modifications regarded as the cornerstone of therapy for diabetes, insists more on regular exercise and proper choice of food. Since these are difficult to uphold, there is a need for a lifestyle intervention for easy and habitual continuation.
Thoroughly chewing a food morsel to form a bolus for swallowing affects post-prandial blood glucose levels as per few studies [7–10]. This is thought to be predominantly due to an increase in cephalic phase of insulin release. Cephalic response has been used traditionally to refer to physiological reflexes that are elicited by stimuli engaging the receptors of the oropharynx or head. Numerous factors like smell, sight of food and duration of sensory stimulation of the oral cavity are well known to cause an increase in the cephalic phase insulin response [11,12]. This study was done to assess the effect of mastication on post prandial blood glucose levels.
Materials and Methods
This study was conducted on 100 healthy volunteers having normal blood glucose values and 100 diabetic patients being treated at a Tertiary Medical Care Centre in South India; having impaired fasting blood glucose/ tolerance values/ those who are known cases of DM; during November 2013-June 2015. This selectively randomized study was conducted after securing Institutional Ethical Committee Clearance, with informed consent of concerned study subjects. Subjects with fasting blood sugar (FBS) ≥100mg/dl or a Post-prandial blood sugar ≥140 mg/dl or glycated haemoglobin(HbA1c) >6.5 mg% were included in dysglycaemic group. While those with FBS< 100 mg/dl and PPBS < 140 mg/dl were recruited in normoglycaemic group.
Subjects with history of recent severe illness, recent surgery, trauma/burns, liver /kidney disease, on alpha glucosidase inhibitors or drugs interfering with actions of insulin (steroids, thiazides) were excluded from the study. Pregnant women and edentulous individuals were disqualified from this study. For this prospective interventional study of two consecutive days, thorough clinical examination of subjects was done. Fasting, pre and postprandial blood sugars were estimated on both days. Other relevant investigations were conducted.
A fixed caloric load (150 Cal in form of 25 grams of groundnuts) was given to each study subject on day 1 and day 2 at same time. On day 1, study subjects were asked to masticate at their normal routine frequency which was counted. On day 2, study subjects were asked to masticate at a fixed frequency of 40 times per bolus before swallowing. For ease of administration, caloric load was given in 3 divided morsels. No changes in anti-diabetic treatment were made on both days of study.
No meals (or snacks) were allowed for at least 2 hours before start of test on both days and in 2 hours between test meal and PPBS, study subjects were told not to consume any meal or engage in any strenuous physical activity and to be preferably at rest. However, they were allowed to drink water, if required.
Statistical Analysis
All data was collected and analysed. With a confidence interval of 95% and 80% power, data was analysed by Student’s paired‘t’ test using SPSS 15.0 software. Probability of less than 5% (p<0.05) was considered to be statistically significant.
Results
This prospective intervention study was conducted on two separate groups, dysglycaemic and normoglycaemic to assess the effect of thorough mastication on PPBS levels. Average mastication frequency on day 1 of normoglycaemics was 29.24 ± 1.73 and of dysglycaemics was 29.08 ± 1.78, while all subjects were made to chew each morsel 40 times on day 2. The mean age values are synchronised in [Table/Fig-1].
Mean age of study subjects.
| Mean age in years |
|---|
| Normoglycaemic (n =100) | 37.93±14.18 |
| Dysglycaemic (n’= 100) | 56.74±11.78 |
The demographic details of all subjects are expressed in [Table/Fig-2,3].
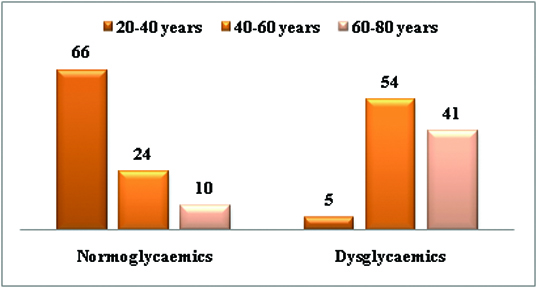
Age-wise distribution of all study subjects.
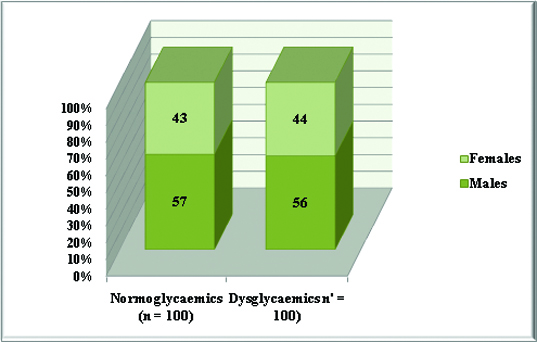
Gender-wise distribution of all study subjects.
The fasting and post-prandial blood sugar values of all subjects were estimated on both days of this selective randomised study as recorded in [Table/Fig-4,5 and 6].
Mean blood sugar values of study subjects*.
| Fasting (pre-prandial) blood sugar values (day 1) | Post-prandial blood sugar values (day 1) | Fasting blood sugar values (day 2) | Post-prandial blood sugar values (day 2) |
|---|
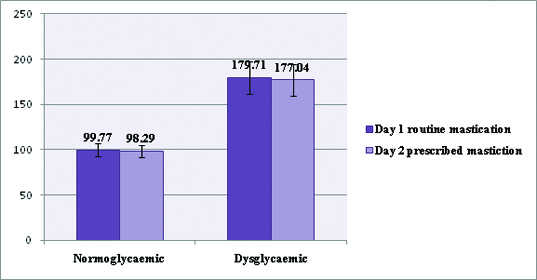
| Normoglycaemic (n =100) | 99.77±7.06 | 128.25±7.82 | 98.29±6.86 | 119.74±9.08 |
| Dysglycaemic (n’= 100) | 179.71±17.8 | 244.07±22.37 | 177.04±17.73 | 243.55±22.87 |
*- p<0.05
Fasting (pre-prandial) blood sugar values of all study subjects on day1and day2.
Post-prandial blood sugar values of all study subjects on day1 and day2.
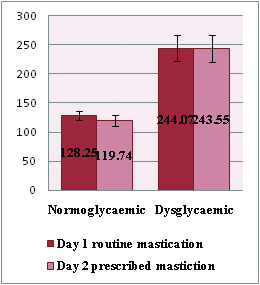
When PPBS levels were estimated on both days of the study i.e. on routine and fixed mastication frequency days, the authors found that the post prandial blood sugar levels reduced significantly in the normoglycaemic group subjects, compared to the dysdglycemic group (p<0.001). However, in the dysglycaemic group, there was statistically less significant difference in PPBS levels on thorough mastication (p>0.05) as depicted in [Table/Fig-6].
Discussion
Mastication is the first step in the process of food metabolism in the body. It breaks down big food particles with nutrients available in complex forms like starch into small pieces, thus increasing its digestibility by increasing surface area for action of various enzymes. This helps in stimulation of cephalic phase of insulin and incretin release from the gut to increase absorption of simple carbohydrates like glucose, etc [13]. In this interventional study to find the effect of thorough mastication on blood sugar levels, authors found significant difference in the PPBS levels on day 1 during routine mastication and on day 2 after thorough mastication (p <0.001) in normoglycaemic group.
Chewing food particles, breaks down complex carbohydrates, proteins and lipids into simpler molecules of glucose, amino acids, fatty acids; these in turn through taste cells and receptors of tongue help in releasing more of insulin independent of plasma glucose values [14] also termed as cephalic phase of insulin release. Mastication is a phenomenon with wide variations in frequency and thoroughness of process across various races, ethnicities, cultures and cuisines. Decreased chewing frequency in fast eaters and subsequent inactivation of neuronal histamine may be related to weight gain [15]. Mastication-induced activation of histamine neurons suppresses physiological food intake through H1-receptors in the hypothalamic paraventricular nucleus and the ventromedial hypothalamic nucleus, known as satiety centres [16–18].
Proper mastication of food can help in controlling excess weight gain and the obesity-related health risk factors. Despite the obvious importance, very few studies have addressed and evaluated the effects of mastication on various parameters of carbohydrate metabolism (blood glucose levels, serum insulin levels, GLP1 levels). Hence, this prospective intervention study was undertaken with the purpose of determining the effect of thorough mastication on postprandial blood glucose levels in dysglycaemic and normoglycaemic individuals.
However, in our study in dysglycaemic group, there was no significant difference in PPBS levels on day 2 after thorough mastication (p>0.05) compared to PPBS values of day 1. One reason for this could be that the dysglycaemic were allowed to continue their hypoglycaemic medication. So the authors would like to suggest a further meticulous study wherein medications of dysglycaemic patients are intervened. Our results echo the findings reported by another similar cross-over trial of 52 test meals in 26 subjects conducted in Japan, where in normoglycaemics, PPBS upon thorough mastication of meal was significantly lower, compared to subjects predisposed to type 2 diabetes (first-degree relatives of type 2 diabetic patients, subjects with impaired glucose tolerance, and type 2 diabetic patients) [9].
Our study, however was conducted on a much larger sample size (100 dysglycaemic and 100 normoglycaemic) compared to previous studies giving us a power of 80% and a confidence interval of 95% in our study [8–11].
Limitation
Our study however has certain limitations. We did not group prediabetics and diabetics separately and in retrospect, authors hypothesize that thorough mastication may have elicited different responses in these 2 subgroups. With our results, population most likely to benefit are both with and without family history of diabetes or other strong risk factors for the development of diabetes like obesity, hypertension, dyslipidaemia etc. Thorough mastication is a simple lifestyle modification and can be very cost effective and habitually sustainable in conjunction with dietary modifications and regular exercise.
Conclusion
Our study showed that thorough mastication significantly reduced postprandial blood sugar levels in normoglycaemics; however, reduction in post-prandial blood sugar levels was not statistically significant in dysglycaemics.
*- p<0.05