Lymphoproliferative malignancies constitute a wide spectrum of haematological malignancies and their prevalence is widely increasing. Non-Hodgkin lymphomas and Hodgkin disease, frequently involve extranodal soft tissue structures in the head and neck, thorax and abdomen. These malignancies may involve virtually any type of soft tissues to any extent; hence many different imaging manifestations are possible which may mimic other disorders.
The imaging characteristics of extranodal lymphomatous soft tissue involvement are described and classified here according to the site of involvement in 6 cases (primary diseases with orbital, muscle, extra testicular, scalp, sinonasal and pachymeningeal/dural involvement). In majority of these cases at presentation we found a predominantly homogeneous soft tissue mass with mildly high attenuation on CT and a T2 intermediate signal on MRI at these sites without any manifestation of disease elsewhere but on follow-up two out of these six cases developed systemic disease elsewhere.
Few consistent patterns were noticed on CT and MRI which might help to include lymphomas as an important differential diagnosis of soft tissue masses. Though a definitive diagnosis requires a biopsy (bone marrow, lymph node, or mass), and other laboratory tests, imaging primarily aims at staging of the disease and identification of new or recurrent disease.
Case Series
The present series include cases of primary soft tissue involvement by lymphoproliferative malignancies with their CT & MRI patterns. These cases were collected over a one year period starting from July 2013 till 2014 and followed up for another one year till July 2015. In all cases systemic disease was ruled out at presentation by staging CT of chest, abdomen and pelvis and bone marrow biopsy. Only those few cases which did not have any manifestation of lymphoma elsewhere have been included in the present series and rest all other cases which showed bone and nodal involvement at presentation on staging CT and bone marrow biopsy have been excluded. [Table/Fig-1] reveals clinical and laboratory parameters of all the 6 cases.
Clinical and laboratory parameters.
| Cases | Age | Sex | Immuno-compromised Status | C/F and location of the mass | Lab Parameters (Cd4,Ldh ) | Hpe Diagnosis | Systemic Disease On Follow Up a |
|---|
| 1.Chest wall lymphoma | 50 y | F | -ve | Insidious onset slowly growing lump involving right axilla and chest wall with oedema of the right arm and occasional fever | CD4-normalLDH+++ | KI positive anaplastic large cell lymphoma | NIL |
| 2.Cranial vault, scalp lymphoma | 40 y | M | HIV +ve | Multiple nodular soft tissue masses over scalp, orbits with weight loss and fever | CD4-90/μL | Diffuse large B cell lymphoma | Succumbed to disseminated Koch’s. |
| 3.Primary orbital lymphoma | 45y | F | -ve | Painless gradually progressive swelling in left orbit with proptosis since last 7 months. No loss of vision initially | CD4- NormalLDH- Normal | Well differentiated nodular lymphocytic lymphoma | Abdominal paraaortic nodes positive on follow up . |
| 4.Primary Dural Lymphoma | 55y | F | -ve | Slowly growing headache, vomiting | Normal | Low grade marginal B cell lymphoma | - |
| 5.Extra testicular lymphoma | 62 y | M | -ve | Left hemiscrotal palpable lump with fever | Normal | Large B cell lymphoma | Abdominal nodes+ve on 6 month follow up |
| 6.Sinonasal lymphoma | 55 y | M | -ve | Left nasal obstruction with headache | Normal | Diffuse large B cell lymphoma | nil |
(LDH-Lactate Dehydrogenase enzyme, HIV-Human Immunodeficiency Virus)
CT was performed in all cases in 40 slice Philips Brilliance scanner with 3mm section thickness 5mm collimation, pitch 1.5, Kv 120, mAs100-120. Raw data was reconstructed to 1mm section thickness in dedicated work station. IV contrast material (iohexol, 300/100, 1.5mg/kg) was administered and all images were studied using both soft-tissue and skeletal window settings.
MRI was performed in 4 out of 6 cases in a 1.5 tesla Siemens Magnetom Symphony scanner with 4mm thick slices and 2mm interslice gap. All standard sequences were performed along with post contrast sequences.
The masses were studied based on the basic pattern involvement, gross and internal morphology, density, heterogeneity on CT scan, signal characteristics on MRI with enhancement pattern and locoregional extension.
Initial staging scan excluded associated systemic disease in these cases but 2 out of 6 of these cases subsequently developed systemic disease.
Majority of our patients had B cell disease and only one of these patients had immuno compromised status [Table/Fig-1]. Majority of these primary lymphomatous masses (5 out of 6 cases) were of homogenous soft tissue attenuation without any necrosis or calcification [Table/Fig-2]. The masses were largely isointense on T1 & mildly hyperintense or intermediate signal on T2, (less than fat and more than muscle) with predominantly homogeneous enhancement on administering contrast. Bone destruction was relatively insignificant compared to the large soft tissue masses [Table/Fig-2]. Only one case of sinonasal lymphoma showed some erosions in the lamina papyracea. There was some bone remodelling changes in the orbital lymphoma but no obvious destruction.
| Cases | Unifocal Mass/Diffusely Infiltrating Type | Attenuation On Ncct | Necrosis/Hemorrage/Calcifications | Mri Signal(T1,T2,Dwi) | Enhancement Characteristics (Ct / Mri) | Vascular/Bone/Visceral Involvement |
|---|
| 1. Chest wall lymphoma | Large unifocal Lobulated mass | NCCT- 45-50 HUCECT-75-90 HU | Occasional areas of necrosis,Subcutaneous stranding ++ | T1-Isointense(with muscle)T2- Mildly hyperintense (compared to muscle)DWI-mild restriction | Relatively homogeneous enhancement with few areas of necrosis | Encasement of right axillary vessels |
| 2. Cranial vault, scalp lymphoma | Diffusely infiltrating masses | NCCT-38-47HU, cect-65-85HU | Dermal and subdermal fat involvement present | T1-iso to hypointenseT2-mildly hyperintenseDWI –restriction +ve | Homogeneous enhancement | -ve |
| 3. Primary orbital lymphoma | Diffusely infiltrating masses in extra and intraconal compartmnet | NCCT-40 -47HU, CECT-80-90 hu | Involvement of skin and eyelids, lacrimal gland | - | Homogeneous enhancement | No bone destruction but some remodeling of the bony orbit |
| 4. Primary Dural Lymphoma | En plaque infiltrating mass along anterior falx | NCCT-50 HU, CECT-80 HU | - | T1- hypontense, T2- hypointenseDWI-restriction | Mild homogeneous enhancement | involvement of sup sagittal sinus |
| 5. Extra testicular lymphoma | infiltrating mass along left epididymis and spermatic cord | NCCT-35-44HU, CECT-78 HU | Secondary involvement of testis | T1-hypointenseT2 hypointenseDWI-not done | Homogeneous intense enhancement | Involvement of left inguinal and pampiniform plexus vessels |
| 6. Sinonasal lymphoma | unifocal Lobulated mass | NCCT-32-46HU, CECT-70-80 HU | -ve | - | homogeneous enhancement | Erosion of lamina papyracea |
Discussion
Primary involvement of extra nodal soft tissue by lymphomas is more common with Non Hodgkins Lymphomas (NHL) than Hodgkins disease [1,2]. As defined by Pinto Leite et al., Lymphoproliferative disorders are a subgroup of haematologic malignancies that comprises of four different malignancy types: Non-Hodgkin lymphomas, Hodgkin disease, lymphocytic leukaemias (acute or chronic), and plasma cell myeloma (multiple myeloma) [2]. With regard to both NHL and Hodgkins the term “extranodal involvement”, both refers to infiltration of tissues other than the lymph nodes, spleen, thymus, tonsils and pharyngeal lymphatic ring except in Hodgkins disease where splenic involvement is considered as nodal [2]. With increasing prevalence of AIDS and immunosuppressive therapies, there is definite increase in involvement of extra nodal structures by lymphomas [2]. However, even with NHL, primary soft tissue involvement is very rare and accounts for only 0.1% of cases [3].
Primary Muscle Lymphoma of the Chest Wall
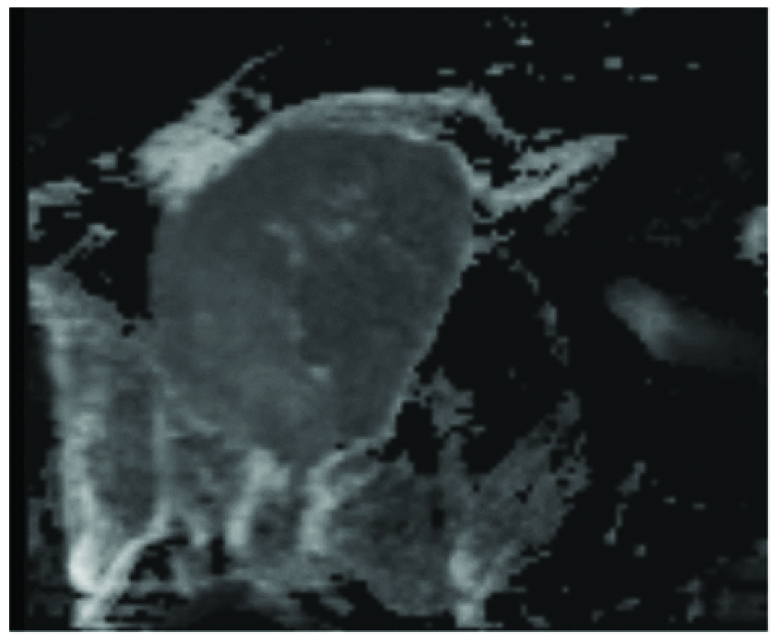
The mass lesion in this 50-year-old was seen to span multiple compartments extending from supraclavicular space superiorly along right antero lateral chest wall muscles involving pectoralis minor into right axillary space [Table/Fig-3,4]. There was evidence of infiltration into the surrounding axillary and subcutaneous fat [Table/Fig-4] as documented by Vivian et al., without any destruction of bones of thoracic cage [4]. Considering the extra thoracic location of the mass, radiological differential diagnoses of rhabdomyosarcoma, lymphoma and breast carcinoma from axillary tail, infiltrating right chest wall were made. Like most cases documented by Chang et al., the mass in our case revealed a T2 intermediate signal [Table/Fig-5] and showed relatively diffuse homogeneous contrast enhancement with occasional areas of necrosis [Table/Fig-6] with low ADC values on ADC map [Table/Fig-7] [5]. Previous literatures documented most cases of primary muscle lymphoma to be arising from skeletal muscles in arms and limbs [4,5] and extra nodal origin of NHL in chest wall muscle is rare [6]. Muscle biopsy revealed anaplastic large cell lymphoma in our case and patient responded to chemotherapy. Follow up staging CT at 6 months and 1 year did not reveal any systemic disease. Previously reported cases were primarily in immunocompromised patients with Diffuse large cell and Burkitt lymphomas as main histopathological diagnoses [6].
CECT chest shows soft tissue mass lesion (red arrow) seen in the supraclavicular space extending into right axilla beneath right pectoralis major.
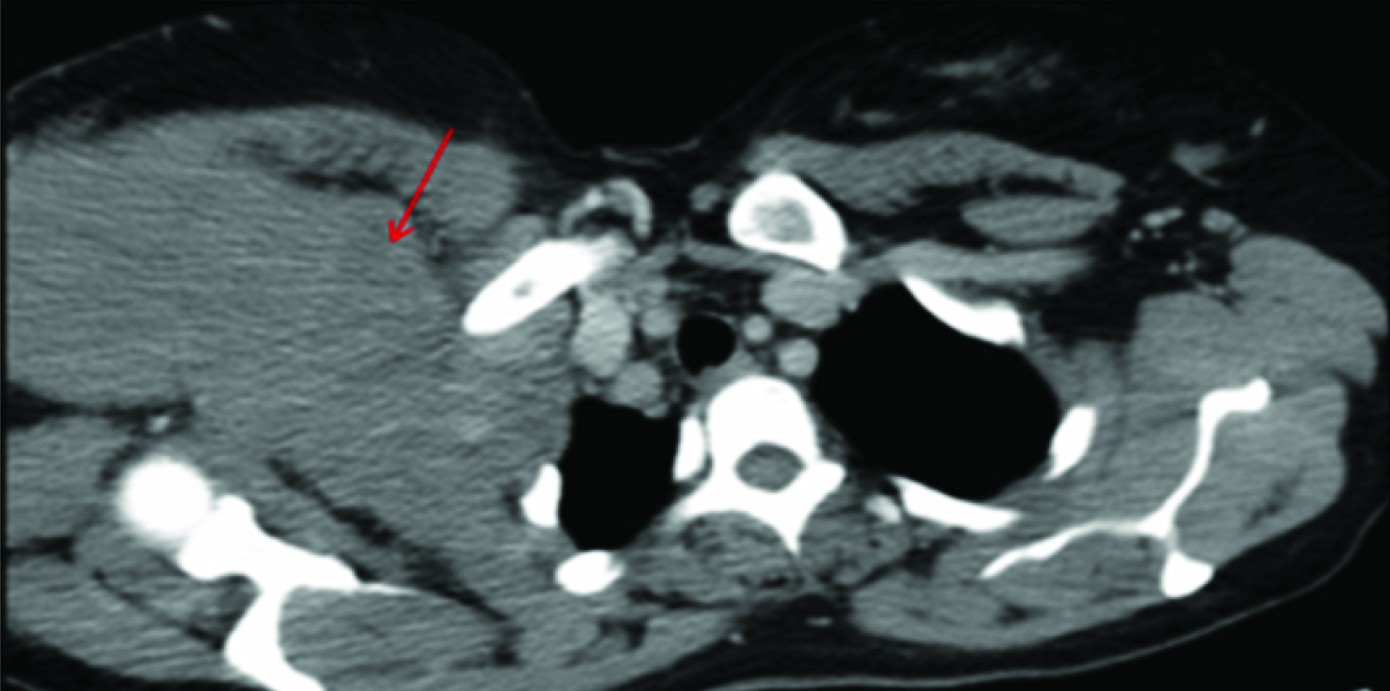
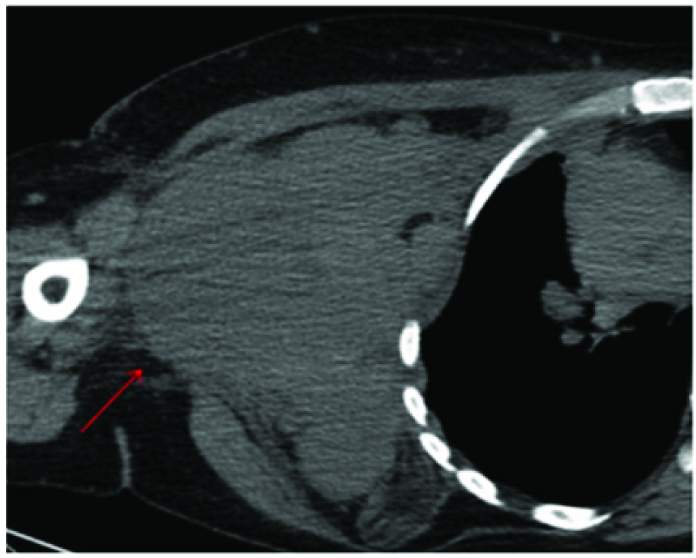
A more caudal axial section of CECT shows some streaking in the subcutaneous fat(red arrow).
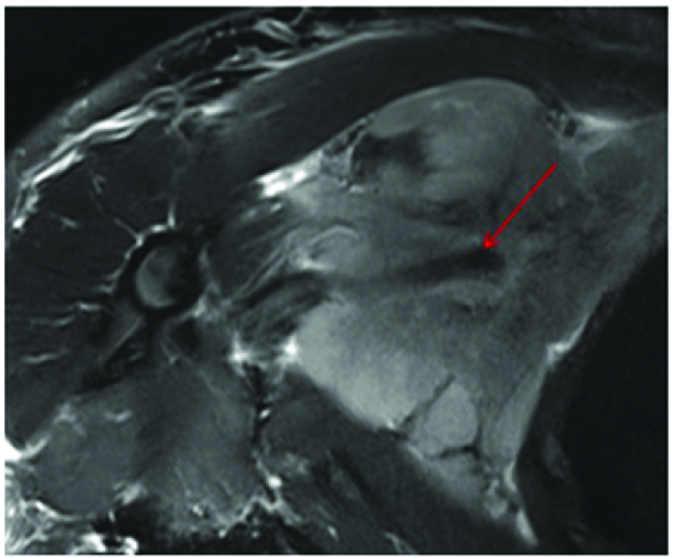
ON T2W image the mass showed intermediate signal (more than muscle) with flow void of axillary vessel seen coursing through it (red arrow).
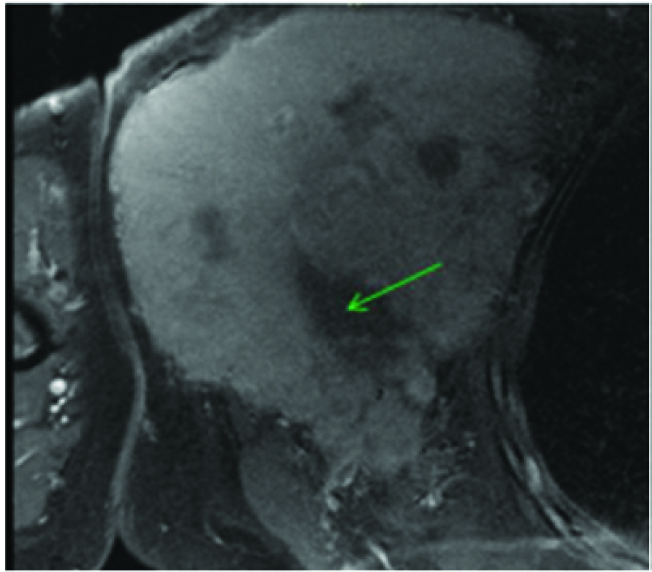
Post contrast T1 FS image showed diffuse homogeneous contrast enhancement with occasional areas of necrosis (green arrow).
The mass showed low ADC values on ADC map.
Primary Cutaneous B-cell Lymphoma of the Scalp and Cranial Vault with Orbital Invasion
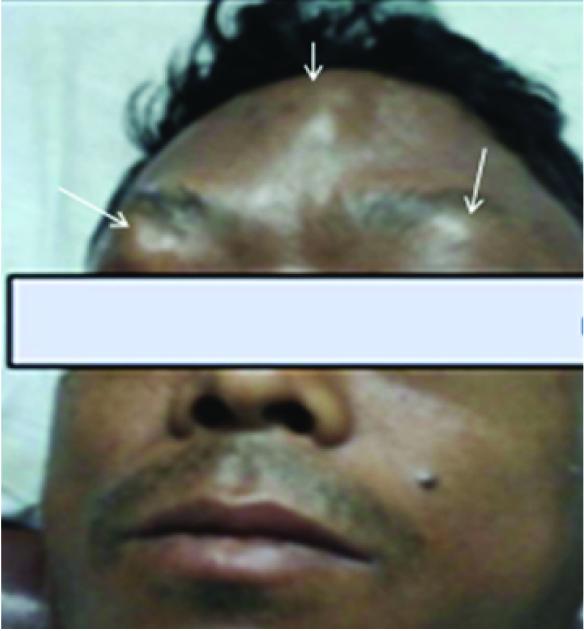
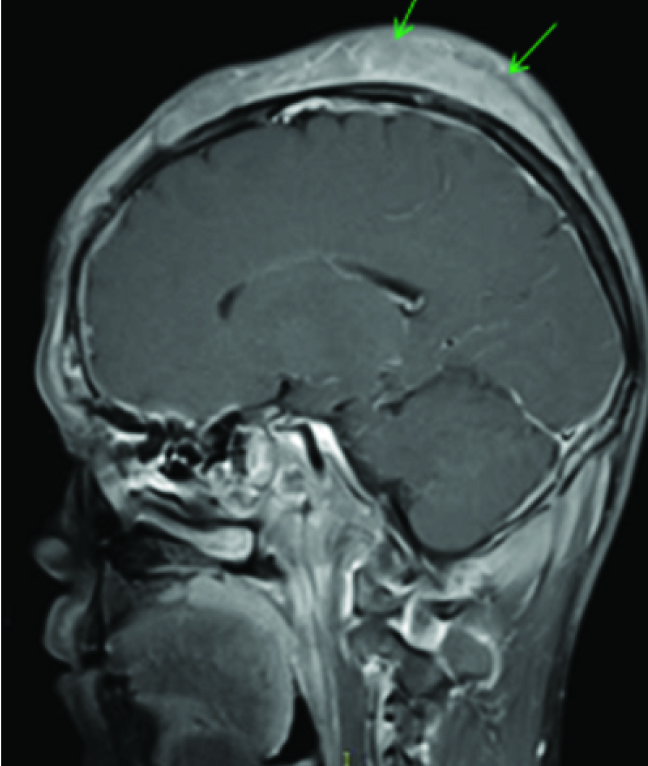
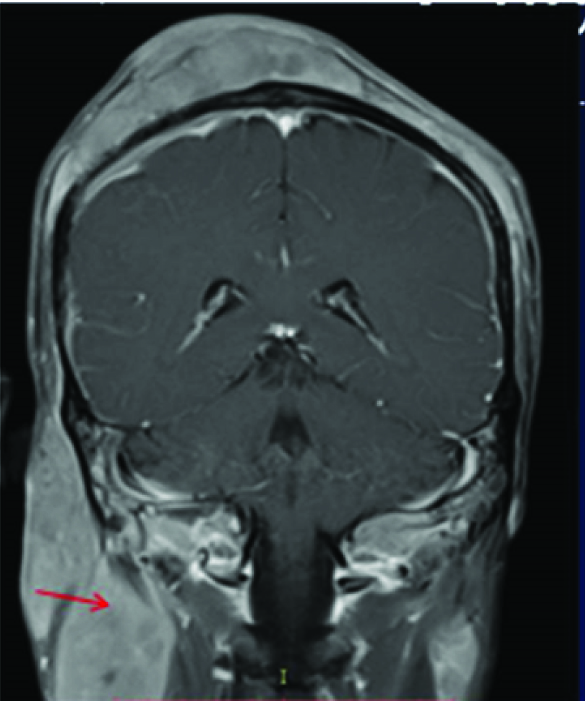
Physical examination of the head and neck in this 45-year-old tea garden worker with retrovirus positive status revealed multiple cutaneous soft tissue masses over the scalp, forehead and eyelids [Table/Fig-8] which on CEMRI showed diffuse enhancement [Table/Fig-9]. There was associated infiltration of the muscles of the infra temporal fossa and masticator spaces [Table/Fig-10], supra orbital and pre septal soft tissues in bilateral orbits. However, there was no associated invasion of the meninges or brain parenchyma or cervical adenopathy. Bone destruction was typically absent in consistent with previously documented cases of primary lymphoma of the cranial vault [7]. Infiltrating and permeating characteristics of lymphoma with large soft tissue component without bone destruction excludes other imaging differential like metastasis from other primary neoplasms, calvarial osteomyelitis. Unlike the case described by Kantarci et al., no invasion of the pachymeninges and brain parenchyma was seen in our case [7]. Head and neck muscles involvement is rare in extra nodal involvement of NHL [8], however extensive involvement of the masticator and pterygoid muscles were seen in the present case which appeared bulky with signal changes and marked contrast enhancement on MRI [Table/Fig-10]. Wedge biopsy from the subcutaneous masses in our case revealed diffuse large B cell lymphoma with CD20 positivity on Immunohistochemistry. Patient was subjected to chemotherapy and HAART but patient succumbed to disseminated pulmonary Kochs.
Multiple palpable cutaneous soft tissue masses(white arrows) were seen over the fore head and eyelids.
Diffusely enhancing soft tissue mass (green arrows) seen over the entire scalp on T1 post contrast images.
Coronal post contrast T1W MR image shows associated enhancing and bulky masticator muscles(red arrow).
Primary Orbital Lymphoma
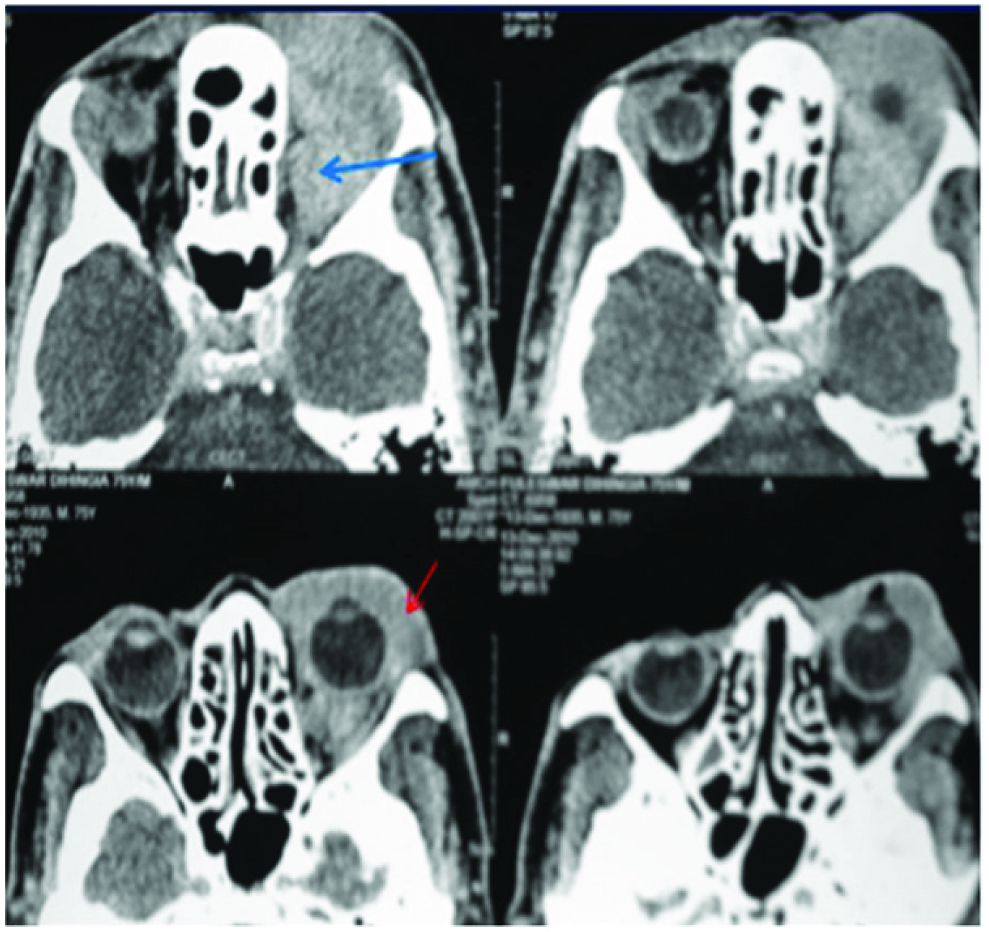
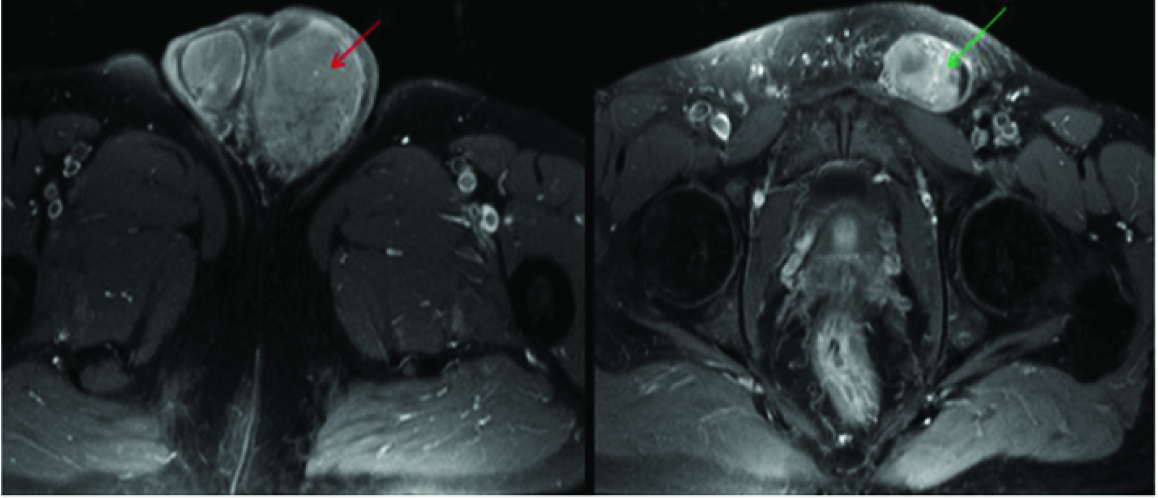
Extra nodal involvement of lymphoma with orbit as primary site is very rare and constitutes 1% of all orbital masses [9]. Exclusion of secondary involvement of orbit in known NHL was done by systemic scan, Chest X ray and bone marrow biopsy at presentation. A diffusely homogeneous mass was seen on CECT involving both extra and intra conal compartment encasing the globe and optic nerve, extending in to the preseptal space [Table/Fig-11]. There was subtle remodelling of bone but no evidence of bone destruction. As there is no specific indicator to differentiate between orbital lymphoma from reactive lymphoid hyperplasia [10], tissue biopsy was done to rule out other imaging differentials like pseudotumour and metastasis which revealed nodular lymphocytic lymphoma. She developed systemic disease on follow-up at 6 months with para aortic nodes in abdominal CT.
Primary Dural Lymphoma
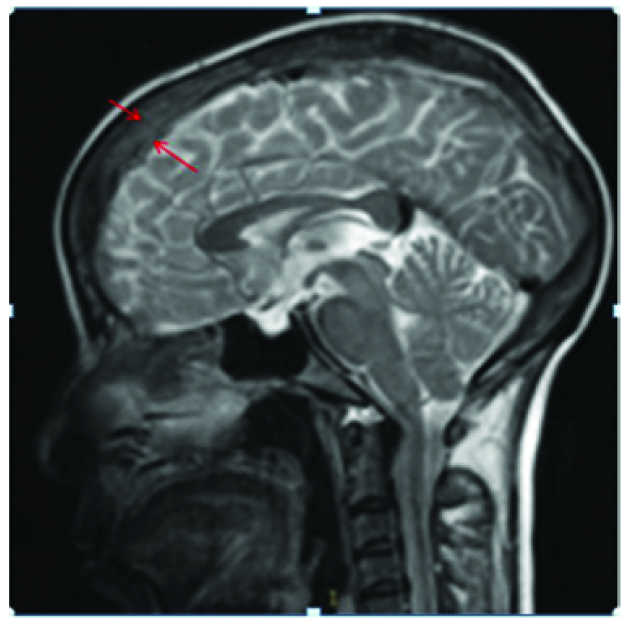
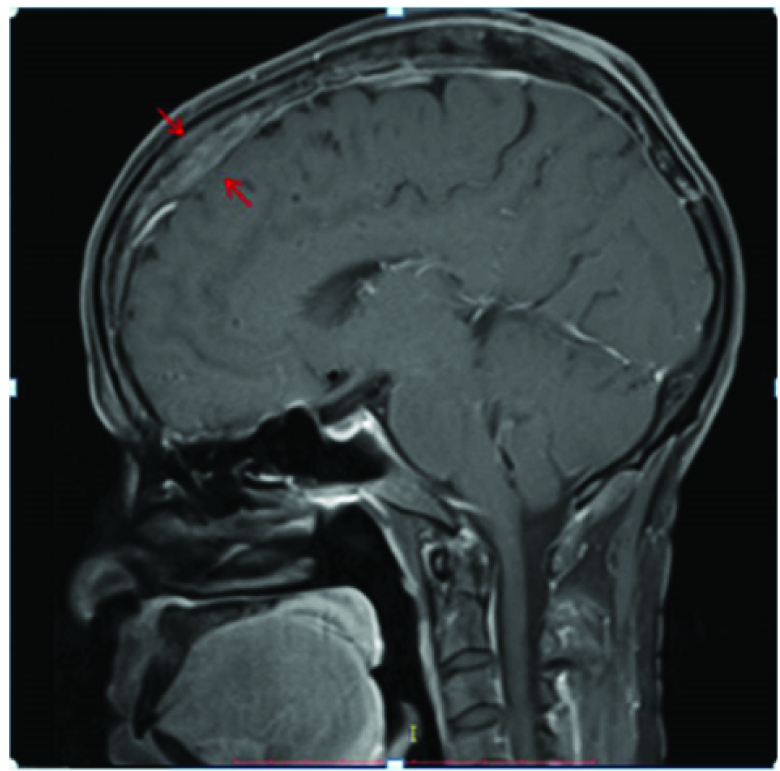
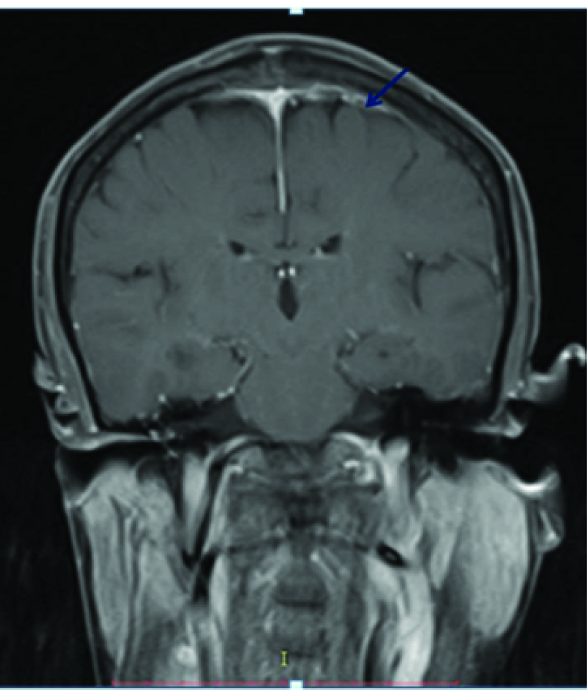
Primary dural lymphoma is extremely rare constituting only 1% of all CNS lymphomas, because the dura is devoid of lymphocytes [11,12]. An enplaque pachymeningeal thickening was seen in our case extending along the superior sagittal sinus with T2 hypointense signal [Table/Fig-12], mild enhancement on postcontrast T1 Wimage [Table/Fig-13] and positive dural tail sign [Table/Fig-14]. Meningioma, dural metastases and primary dural lymphoma were considered as differentials. An extensive search for primary and systemic disease was excluded by staging CT and bone marrow aspirate. However, no underlying parenchymal invasion was seen in our case and the patient subsequently underwent resection with radiotherapy but was lost to follow-up. Though a study [13] reported more than 50% cases with multiple metachronous lesions in brain as well as spine, no such findings were seen in our case. In contrast to primary CNS lymphoma, Primary dural lymphoma has a better prognosis [12,13].
Diffusely homogeneous mass was seen on CECT involving both extra and intra conal compartment encasing the globe and optic nerve (blue arrow), extending in to the preseptal space (red arrow).
An enplaque pachymeningeal thickening with T2 hypointense signal seen along the superior sagittal sinus (red arrows)
Mild enhancement of the lesion seen on post contrast T1W image (red arrows). No underlying parenchymal or calvarial invasion is seen.
A positive dural tail sign was seen on post contrast MR (blue arrow).
Primary Extratesticular Lymphoma
Genital tract lymphomas predominantly involve the testis but are known to involve the paratesticular structures like epididymis and spermatic cord with a reported frequency of 60% and 40% respectively [14,15]. The mass lesion in our patient extended along the left epididymis and spermatic cord with diffuse thickening of these structures [Table/Fig-15,16]. Testis was displaced postero inferiorly with preserved signal [Table/Fig-16]. Diffuse homogeneous enhancement of the left epididymis and cord was seen on post contrast study [Table/Fig-17]. As no fat signal was appreciated in the mass lesion, imaging differentials of leiomyosarcoma, adenocarcinoma of epididymis, and even chronic tuberculosis and sarcoidosis of epididymis were considered. However, peroperative findings revealed secondary infiltration of the left testis and postoperative biopsy showed diffuse large B cell lymphoma which is the most common type of testicular lymphoma seen in aged adults after 60 years. Thus the primary origin of the lymphoma to testis/epididymis could not be localized with certainity however on imaging the lesion was primarily seen along the epididymis and spermatic cord. To the best of our knowledge there is hardly any reported case of extratesticular lymphoma involving epididymis primarily [16,17]. On follow-up, systemic disease with positive retroperitoneal nodes were detected on abdominal CT in our patient.
Coronal T2 hypointense mass seen along left epididymis (red arrow)and spermatic cord (green arrow) with diffuse thickening of these structures. Left Testis was compressed and pushed postero medially with preserved signal (red dotted circle). There was some hydrocele on right side.
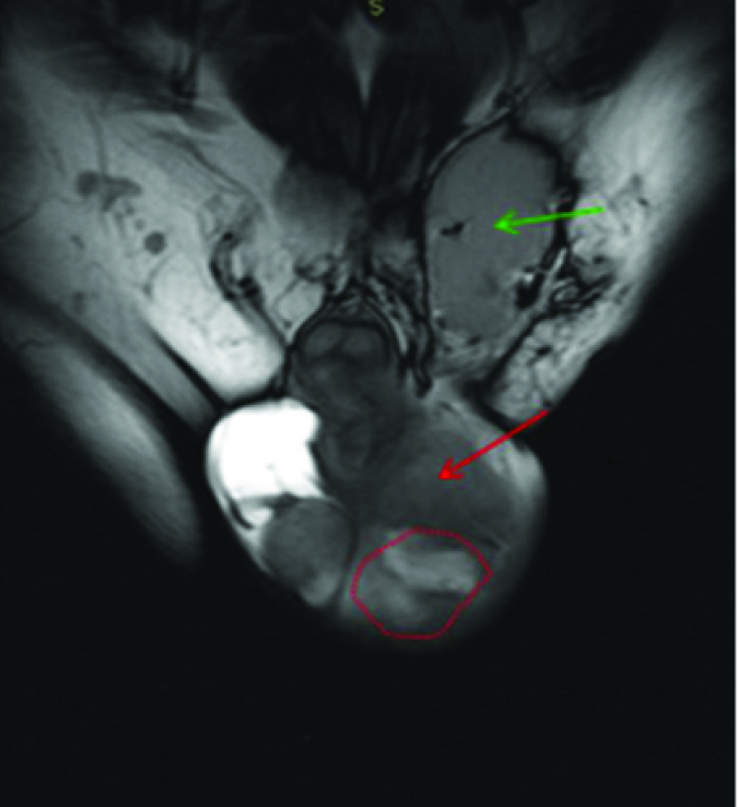
Axial T2 W image shows hypointense mass involving left epididymis (red arrow) with diffuse thickening. Left Testis seen in more caudal section shows preserved signal (red dotted circle). There was some hydrocele on right.
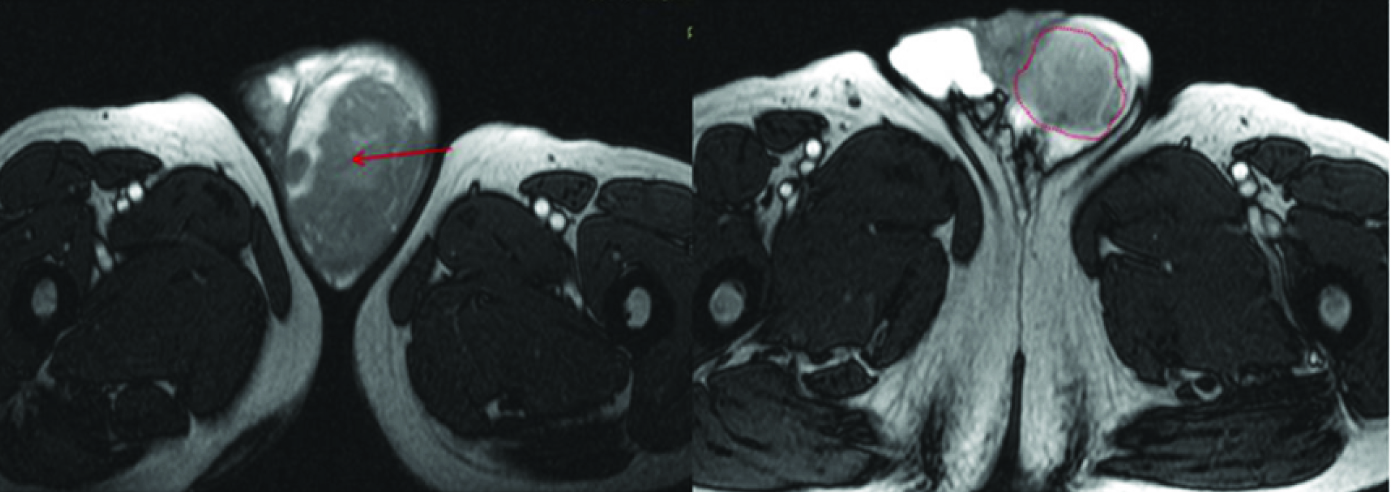
Axial T1 W post contrast image shows diffusely enhancing mass involving left epididymis (red arrow). Thickening with enhancement of the cord seen on left side in a superior section.
Primary Sinonasal Lymphoma
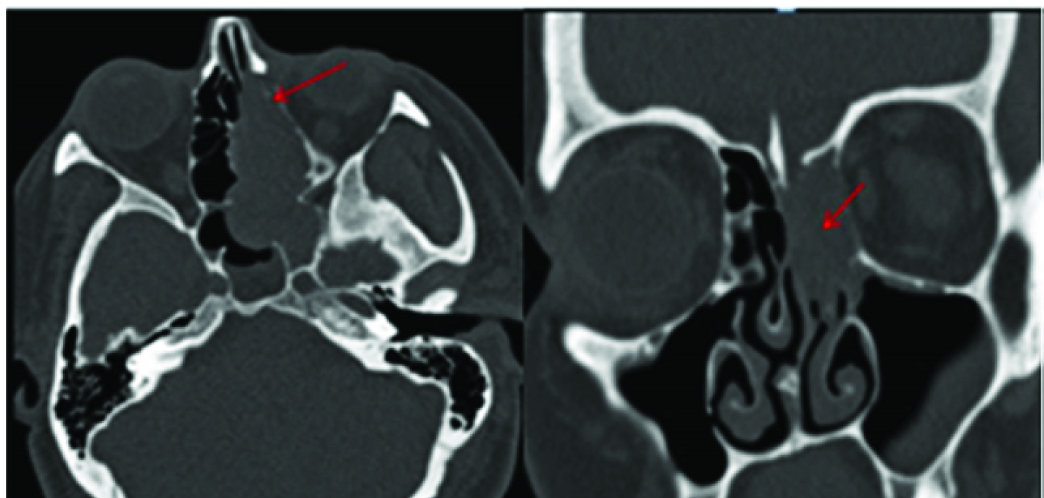
Sinonasal involvement is one of the most common sites of extra nodal involvement of NHL in Head and Neck [18]. Among the sinonasal lymphomas, Diffuse large cell lymphoma is the most common followed by NK/T cell lymphoma [16,17]. In our patient the mass was restricted to the left half of nasal cavity with evidence of significant enhancement on post contrast study [Table/Fig-18] and erosion of the left sided turbinates and lamina papyracea on CT [Table/Fig-19a&b]. Histopathology showed Diffuse Large B Cell Lymphoma (DLBL) unlike that reported by Garcia et al and Zagólski et al., where most DLBL showed associated involvement of sinuses [18,19], whereas NK/T cell lymphoma were mostly restricted to nasal cavity with erosion of septum or turbinates [20]. In our patient, the mass was seen to cause blockage of left osteomeatal complex with accumulated secretion in left maxillary antrum but no invasion. Sinonasal lymphoma can resemble any other inflammatory and benign masses and tissue biopsy is extremely important to initiate the management [21] as there is no specific radiological hallmark but it should be considered as an important differential.
A Lobulated soft tissue dense mass (red arrows) seen in left half of nasal cavity.
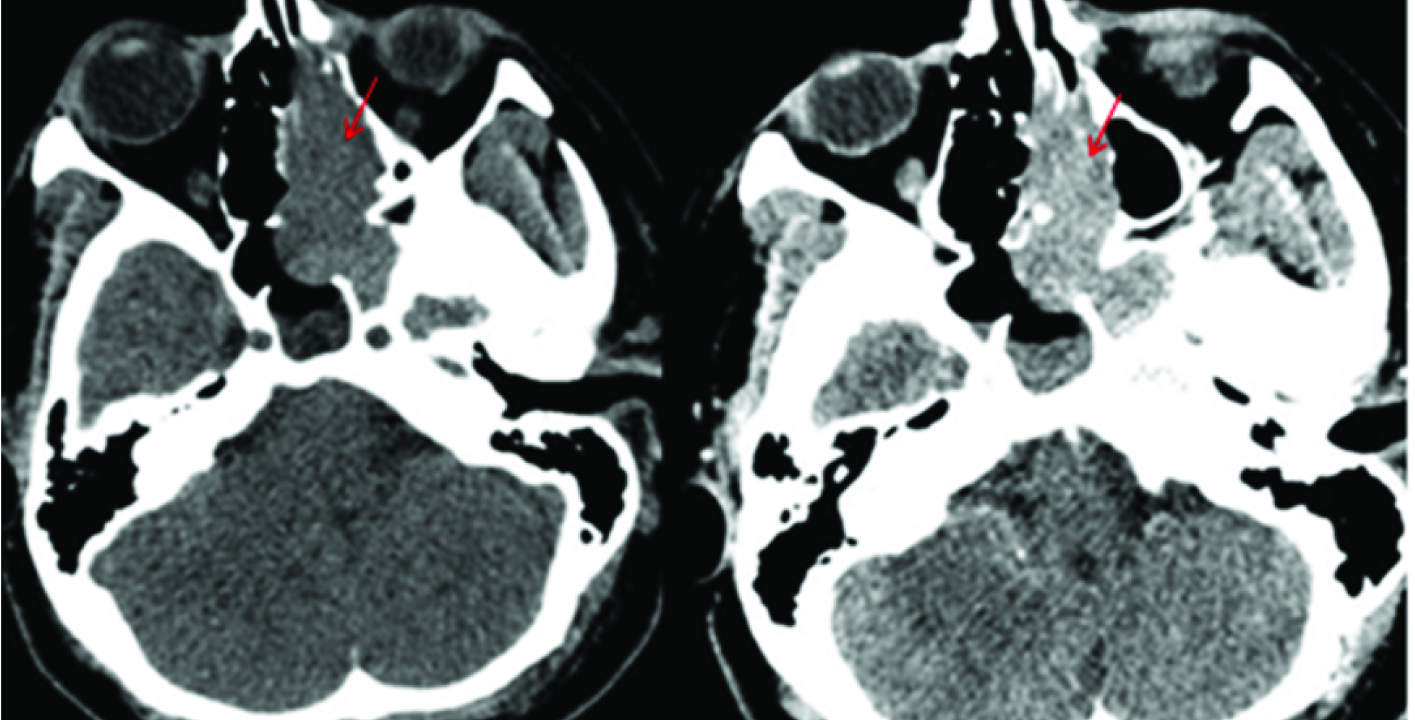
Erosion of the left sided turbinates and lamina papyracea (red arrows) seen on bone window images in CT.
Conclusion
Besides describing the radiographic manifestations of extra nodal soft tissue involvement by lymphoproliferative malignancies at atypical locations, this case series intended to determine whether such extranodal involvement represents a primary manifestation or dissemination of systemic disease, which has a poorer prognosis and finally to find out any specific or consistent radiographic features to differentiate from other tumour mimics, infections.
Most cases of primary extra nodal lymphoma in the series belonged to the B cell group with few consistent imaging patterns were noted like homogeneous enhancing soft tissue masses with remarkably minimal bone destruction, a T2 intermediate signal on MRI with restricted diffusion of high cellularity lesions. A familiarity with these features may help to include lymphoma as an important differential and establish a quick diagnosis. Baseline systemic scan is important to ascertain whether extranodal involvement represents a primary disease or secondary manifestation of systemic disease, which has a poorer prognosis.
(LDH-Lactate Dehydrogenase enzyme, HIV-Human Immunodeficiency Virus)
[1]. Guermazi A, Brice P, de Kerviler E, Ferme C, Extranodal hodgkin disease: spectrum of diseaseRadio Graphics 2001 21:161-79. [Google Scholar]
[2]. Leite NP, Kased N, Hanna RF, Brown MA, Cross-sectional Imaging of extranodal involvement in abdominopelvic lymphoproliferative malignanciesRadioGraphics 2007 27:1613-34. [Google Scholar]
[3]. Corti M, Bistmans A, Narbaitz M, Soft-tissue masses as presentation of non-Hodgkin’slymphoma in AIDS patientsAn Bras Dermatol 2013 88(4):631-34. [Google Scholar]
[4]. Lee VS, Martinez L, Coleman E, Primary muscle lymphoma: clinical and imaging findingsRadiology 1997 203:237-44. [Google Scholar]
[5]. Chun CW, Jee W-H, Park HJ, Kim YJ, MRI features of skeletal muscle lymphomaAJR 2010 195:1355-60. [Google Scholar]
[6]. Zaleski CG, Abdenour GE, Pediatric case of the dayRadio Graphics 1997 17:227-31. [Google Scholar]
[7]. Kantarci M, Erdem T, Alper F, Gundogdu C, Imaging characteristics of diffuse primary cutaneous b-cell lymphoma of the cranial vault with orbital and brain invasionAJNR 2003 24:1324-26. [Google Scholar]
[8]. Chong J, Som PM, Silvers AR, Dalton JF, Extranodal non-hodgkin lymphoma involving the muscles of masticationAJNR 1998 19:1849-51. [Google Scholar]
[9]. Mills P, Parsons CA, Primary orbital lymphoma: staging by computed tomographic scanningThe British Journal of Radiology 1989 62:287-89. [Google Scholar]
[10]. Westacott S, Garner A, Moseley IF, Wright JE, Orbital lymphoma versus reactive lymphoid hyperplasia: an analysis of the use of computed tomography in differential diagnosisBritish Journal of Ophthalmology 1991 7:722-25. [Google Scholar]
[11]. Knipe H, Gaillard F, Primary dural lymphomaRadiopaedia.org [internet] [updated 2002 May 16; cited September 2015] Available from http://radiopaedia.org/articles/primary-dural-lymphoma [Google Scholar]
[12]. Iwamoto FM, Abrey LE, Primary dural lymphomas: a reviewNeurosurg Focus 2006 21(5):E5 [Google Scholar]
[13]. Iwamoto FM, DeAngelis LM, Abrey LE, Primary dural lymphomas:a clinicopathologic study of treatment and outcome in eight patientsNeurology 2006 66:1763-65. [Google Scholar]
[14]. Doll DC, Weiss RB, Malignant lymphoma of thetestisAm J Med 1986 81:515-24. [Google Scholar]
[15]. Beccia DJ, Krane RJ, Olsson CA, Clinical management of non-testicular intrascrotal tumoursJ Urol 1976 116:476-79. [Google Scholar]
[16]. Ulbright TM, Amin MB, Young RH, Miscellaneous primary tumours of the testis, adnexa, and spermatic cord. In: Rosai J, Sobin LH, edsAtlas of tumour pathology, fasc 25, ser 3 1999 Washington, DCArmed Forces Institute of Pathology:235-366. [Google Scholar]
[17]. Phillips G, Kumari-Subaiya S, Sawitsky A, Ultrasonic evaluation of the scrotum in lymphoproliferative diseaseJ Ultrasound Med 1987 6:169-75. [Google Scholar]
[18]. Zagólski O, Dwivedi RC, Subramanian S, Kazi R, Non-Hodgkin’s lymphoma of the sino-nasal tract in childrenCancer Journal 2010 6(1):5-10. [Google Scholar]
[19]. Garcia C, Proulx GM, Wu CL, Wang CC, Pilch BZ, Harris NL, Sinonasal lymphoma: a clinicopathologic analysis of 58 cases from the massachusetts general hospitalAm J Surg Pathol 1999 23(11):1356-69. [Google Scholar]
[20]. Lee HJ, Im J-G, Goo JM, Kim KW, Peripheral T-Cell Lymphoma: Spectrum of Imaging Findings with Clinical and Pathologic FeaturesRadio Graphics 2003 23:7-28. [Google Scholar]
[21]. Peng KA, Kita AE, Suh JD, Bhuta SM, Sinonasal lymphoma: case series and review of the literatureAllergy and Rhinology 2014 4(8):670-74. [Google Scholar]