Unusually Aggressive Primary Testicular Diffuse Large B Cell Lymphoma with Post Therapy Extensive Metastasis
Shalini Goel1, Ritesh Sachdev2, Ishani Mohapatra3, Smeeta Gajendra4, Sunil Gupta5
1 Associate Consultant, Department of Pathology and Laboratory Medicine, Medanta- The Medicity Hospital, Sector 38, Gurgaon, India.
2 Senior Consultant, Department of Pathology and Laboratory Medicine, Medanta- The Medicity Hospital, Sector 38, Gurgaon, India.
3 Consultant, Department of Pathology and Laboratory Medicine, Medanta- The Medicity Hospital, Sector 38, Gurgaon, India.
4 Associate Consultant, Department of Pathology and Laboratory Medicine, Medanta- The Medicity Hospital, Sector 38, Gurgaon, India.
5 Associate Consultant, Department of Medical Oncology and Hematology, Medanta- The Medicity Hospital, Sector 38, Gurgaon, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ritesh Sachdev, Senior Consultant, Department of Pathology and Laboratory Medicine, Medanta – The Medicity, Sector 38, Gurgaon – 122001, India.
E-mail: sachdev05@gmail.com
Primary Testicular Lymphoma (PTL) is a rare intermediate to high grade tumour, diffuse large cell being the most common type. Unlike nodal Diffuse Large B-Cell Lymphoma (DLBCL), testicular DLBCL has a less aggressive course and better prognosis. Metastasis is uncommon in testicular DLBCL. Commonly involved sites are contralateral testes, Waldeyer’s ring, skin, lung, Central Nervous System (CNS) and prostate, however the kidneys, liver, bone marrow, pleura and bones are more rarely involved. We report a case of testicular DLBCL which has metastasized to skin and bone marrow with an aggressive clinical course in a year, in-spite of combined modality of therapy given to the patient. Bone marrow infiltration is common and well documented with nodal DLBCL, however there is no published literature for simultaneous bone marrow and skin infiltration in testicular DLBCL till date. Other large studies done in the west have shown that distinct metastasis is usually common but the median progression-free survival is usually in years. This case stresses on shorter period of progression after standard treatment protocol in this part of the world, thus highlighting the need for other extensive studies to define specific treatment protocol for testicular DLBCL.
CNS metastasis, Lung metastasis, Positron Emission Tomography-Computed Tomography (PET-CT)
Case Report
A 72-year-old-male presented with right testicular mass of 4 months duration with no history of fever, weight loss or loss of appetite; for which right orchidectomy was done. Patient came to our institute post-surgery for further management. Positron Emission Tomography-Computed Tomography (PET-CT) was done, which revealed post-surgical changes in the right inguinal-scrotal region with no other focus of abnormal hyper metabolism in rest of the body. The bone marrow was cellular with no evidence of any involvement by lymphoma. Cerebro-Spinal Fluid (CSF) cytology was reported negative for malignant cells. The patient tested negative for Human immunodeficiency virus and hepatitis C virus. The histopathological slides of testicular mass were reviewed and showed diffuse infiltration by sheets of predominantly large cells with vesicular chromatin and prominent nucleoli [Table/Fig-1a]. The cells were positive for CD45 (Dako, 2B11+PD7/26), CD20 (Dako, L26), focal positive for MUM 1 (Dako; MUM1p) with Ki-67 labeling index of 60% and negative for CD3 (Dako, IS503), CD10 (Dako; 56C6), Bcl-6 (Dako; PG-B6P), CD30 (Dako; Ber-H2) and CK (Dako, AE1+AE3) [Table/Fig-1b]. Thus, a diagnosis of B-cell NHL favoring DLBCL of non-germinal center B-cell type was given. Patient was clinically staged using the Ann Arbor staging system as Stage IAE (Tumour limited to single extranodal site with no constitutional symptoms). He was started on standard therapy for DLBCL comprising of 6 cycles of R-CHOP (Rituximab, Cyclophophamide, Adriamycin, Vincristine and Prednisolone) followed by intrathecal methotrexate. He completed his chemotherapy, followed by IFRT (Involved Field Radiotherapy) to the scrotum and contralateral testes to the total dose of 3060 cGy in 17 fractions. He was on regular follow up following completion of therapy.
(a) PET CT scan showing multiple FDG avid nodules involving the medial aspect of thigh [coronal MIP image; coronal image (lower left) and axial image (lower right)]. (b) Testicular biopsy shows replacement by monomorphic sheets of large cells (H&Ex400) with prominent nucleoli (Inset; H&Ex1000). IHC markers; positive for CD45, CD20, focal positivity for MUM-1 and negative for CD10 & CD3, Ki-67 index 60% (x 1000). (c&d) Skin and bone marrow biopsy shows infiltration by large B cells (H&E x 200) which are CD20 positive (inset;x1000).
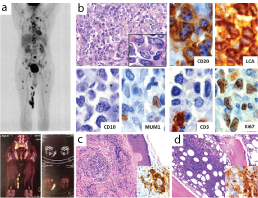
After a year, he presented with multiple hard and discrete nodules in the medial aspect of right thigh. PET-CT showed hyper metabolic multiple soft tissue nodules involving the subcutaneous plane of medial aspect of thigh (proximal and lower aspect adjacent to right knee). Lymphomatous hepatic (caudate lobe) and multiple hyper metabolic skeletal lesions were also noted [Table/Fig-1b]. There was mild increase in alkaline phosphatase and gamma glutaryl transferase (GGT) levels. Peripheral blood findings (Haemoglobin: 14.2 g/dl, Platelets: 2, 56,000/cu mm and Total Leucocyte Count: 8,600/cu mm) were normal with peripheral smear showing few reactive lymphocytes. Histopathology of the skin nodules revealed infiltration by abnormal lymphoid cells with similar morphology as mentioned previously. The cells were positive for CD 20 with Ki-67 labeling index of 50%, indicative of cutaneous metastasis by a B cell lymphoma [Table/Fig-1c]. Simultaneously, bone marrow biopsy also showed CD 20 positive [Table/Fig-1d] lymphoma infiltration with surrounding areas showing fibrosis. The patient was started on R-DHAP (Rituximab, Dexamethasone, Cytarabine and Cisplatin). On further follow up, after two months the patient presented with extensive CNS and lung metastases. The patient was discharged against medical advice and no further follow up was documented.
Discussion
Primary testicular lymphoma is a rare tumour accounting for 1-7% of all testicular neoplasms and 1% of Non-Hodgkin’s lymphoma (NHL) [1,2]. It is usually an intermediate or high-grade tumour which is commonly of diffuse large-cell type [3] with Non-Germinal Center B-cell type (Non GCB) being the most common [4]. The various aetiological factors in the pathogenesis of testicular DLBCL include trauma, cryptorchidism, chronic orchitis and filariasis. The HIV positivity is associated with increased risk of primary testicular lymphomas and increased incidence in younger patients. As mentioned above, the Non GCB type of DLBCL is more common than GCB type. The pathogenesis of GCB phenotype show t (14:18) while bcl2 is expressed in majority of DLBCL irrespective of the phenotype. It suggests the antiapoptotic functions of bcl2. Apart from that bcl6 which is a transcriptional repressor genes, so when mutated in DLBCL, it plays a role in lymphomagenesis by altering B cell terminal differentiation. NF-kB activation is also important particularly in GCB type [5].
The present case presented with painless, unilateral swelling with no associated B symptoms with the presenting duration of 4 months, similar to the other studies done in Indian subcontinent [6]. The diagnosis of Testicular DLBCL poses a challenge not only because of the rarity of the disease (~7% of testicular neoplasms) but also due to the aggressive nature of the disease. The diagnosis involves morphology which can resemble other neoplastic and non-neoplastic conditions such as Seminoma, embryonal carcinoma, plasmacytomas and granulomatous orchitis. In neoplastic conditions such as seminoma, there is monotonous population of cells with round nucleus and moderate amount of eosinophilic cytoplasm. In embryonal carcinoma, there are epithelioid like cells arranged in various patterns such as glandular are seen. On histomorphology, testicular DLBCL is composed of diffuse pattern of large cells which resemble either centroblasts or immunoblasts. The cells sometimes are anaplastic with neutrophilic infiltration, seen in diffuse pattern totally replacing the normal tissue. In plasmacytomas, the cells predominantly show eccentric nucleoli with perinuclear hoff and round nuclei arranged in sheets. Morphologically in inflammatory conditions such as granulomatous or viral orchitis, presence of reactive lymphoid tissue is common however; diligent search for presence of granulomas and viral inclusions can help in reaching to the diagnosis. Immunohistochemistry can further help in confirming the diagnosis of DLBCL with CD45, CD20, focal MUM - 1 positivity and high Ki index, thus distinguishing them from seminoma and embryonal carcinoma (lack of CK expression in the tumour cells).
Testicular DLBCL shows dissemination to certain extranodal sites, which include the contralateral testis, Waldeyer’s ring, skin, lung, and central nervous system (CNS) [4,7]. The other sites are bone marrow, soft tissue, adrenal glands, liver, gastrointestinal tract, and spleen [8]. Involvement of extranodal sites at the time of diagnosis has been previously reported in the literature. They usually have a poor prognosis; however the prognosis is better than nodal DLBCL [9]. Testicular DLBCL has particularly a high risk of extranodal relapse even in cases with localized disease at diagnosis [8]. Involvement of bone marrow although rare, at the time of diagnosis (~5%) has been well documented in the literature [8]. In the present case, no hypermetabolic focus suggestive of metastases was noted at the time of diagnosis in PET- CT scan. The diagnostic bone marrow was also noted to be free of infiltration. The patient was staged as Stage IAE. However, metastasis to the skin and bone marrow occurred a year post treatment which was confirmed by PET-CT scan, skin biopsy and bone marrow examination, thus the stage progressed to Stage IV. In the study by Zucca et al., most of the patients presented with localized disease (stage I or II) with overall median survival rate of 4.8 years and median progression-free survival of 4 years [8]. According to one of the largest cohort studies done by Jacob et al., the disease free survival rate has been reported to be 3 years in 71.5% cases [9]. In another institutional series from India, one case of testicular DLBCL presenting with Stage IE was reported with the patient staying in remission for 78 months. The present case progressed from Stage I to Stage IV in a year from the time of diagnosis, post standard treatment. In the same study [9] CNS involvement had not been noted with bone marrow involvement, yet in the present case there has been involvement of multiple organs in the body including skin, lung, bone marrow and CNS simultaneously and/or subsequently within a short period of time [6].
Due to the aggressive nature and rapid progression of testicular DLBCL, timely and correct diagnosis is of importance for the start of treatment.
Conclusion
To the best of our knowledge this case is rare, in which testicular DLBCL metastasized simultaneously to bone marrow and skin with further involvement of CNS and lung post completion of standard treatment of DLBCL. This case has also documented the progression of the disease from Stage I to Stage IV in a span of a year, where most of the studies have given a higher median survival rate. There is no treatment protocol specific to testicular DLBCL and various studies have proposed different treatment protocols.
[1]. Gunera SI, Karacetinb D, Yukselc M, Primary testicular diffuse large b-cell lymphoma: a case reportWorld J Oncol 2013 4(1):61-5. [Google Scholar]
[2]. Bhatia K, Vaid AK, Gupta S, Doval DC, Talwar V, Primary testicular non-Hodgkin’s lymphoma – a review articleSao Paulo Med J 2007 125(5):266-68. [Google Scholar]
[3]. Gundrum JD, Mathiason MA, Go RS, Moore DB, Adult diffuse large B cell lymphoma of the testis: Analysis of the surveillance Epidemiology and end results data base from 1980-2004J Clin Oncol 2008 26:19524 [Google Scholar]
[4]. Al-Abbadi MA, Hattab EM, Tarawneh MS, Amr SS, Orazi A, Ulbright TM, Primary testicular diffuse large B-cell lymphoma belongs to the nongerminal center B-cell-like subgroup: a study of 18 casesModern Pathology 2006 19:1521-27. [Google Scholar]
[5]. Bacon CM, Freeman A, Urological Cancers: Small cell tumours, lymphomas, and sertoli cell and leydig cell tumours of the bladder, prostate and testis 2005 LondonSpringer Science & Business Media [Google Scholar]
[6]. Gupta D, Sharma A, Raina V, Bakhshi S, Mohanti BK, Primary testicular non-Hodgkin lymphoma: A single institution experience from IndiaIndian J Cancer 2009 46(1):46-49. [Google Scholar]
[7]. Horne MJ, Adeniran AJ, Primary diffuse large B-cell lymphoma of the testisArch Pathol Lab Med 2011 135(10):1363-67. [Google Scholar]
[8]. Zucca E, Conconi A, Mughal TI, Sarris AH, Seymour JF, Vitolo U, International Extranodal Lymphoma Study Group. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study GroupJ Clin Oncol 2003 21(1):20-7. [Google Scholar]
[9]. Gundrum JD, Mathiason MA, Moore DB, Go RS, Primary testicular diffuse large b-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximabJ Clin Oncol 2009 27(31):5227-32. [Google Scholar]